Legend or Truth: Mature CD4+CD8+ Double-Positive T Cells in the Periphery in Health and Disease
- PMID: 37893076
- PMCID: PMC10603952
- DOI: 10.3390/biomedicines11102702
Legend or Truth: Mature CD4+CD8+ Double-Positive T Cells in the Periphery in Health and Disease
Abstract
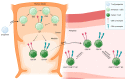
The expression of CD4 and CD8 co-receptors defines two distinct T cell populations with specialized functions. While CD4+ T cells support and modulate immune responses through different T-helper (Th) and regulatory subtypes, CD8+ T cells eliminate cells that might threaten the organism, for example, virus-infected or tumor cells. However, a paradoxical population of CD4+CD8+ double-positive (DP) T cells challenging this paradigm has been found in the peripheral blood. This subset has been observed in healthy as well as pathological conditions, suggesting unique and well-defined functions. Furthermore, DP T cells express activation markers and exhibit memory-like features, displaying an effector memory (EM) and central memory (CM) phenotype. A subset expressing high CD4 (CD4bright+) and intermediate CD8 (CD8dim+) levels and a population of CD8bright+CD4dim+ T cells have been identified within DP T cells, suggesting that this small subpopulation may be heterogeneous. This review summarizes the current literature on DP T cells in humans in health and diseases. In addition, we point out that strategies to better characterize this minor T cell subset's role in regulating immune responses are necessary.
Keywords: CD4+CD8+ T cells; T cell development; T cell differentiation; adaptive immunity; aging; viral infections.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Canine peripheral blood CD4+CD8+ double-positive Tcell subpopulations exhibit distinct Tcell phenotypes and effector functions.Vet Immunol Immunopathol. 2017 Mar;185:48-56. doi: 10.1016/j.vetimm.2017.01.005. Epub 2017 Jan 24. Vet Immunol Immunopathol. 2017. PMID: 28242002
-
Extrathymic CD4/CD8 double positive T cells.Vet Immunol Immunopathol. 1999 Dec 15;72(1-2):55-66. doi: 10.1016/s0165-2427(99)00118-x. Vet Immunol Immunopathol. 1999. PMID: 10614493 Review.
-
Canine CD4(+)CD8(+) double-positive T cells can develop from CD4(+) and CD8(+) T cells.Vet Immunol Immunopathol. 2014 Dec 15;162(3-4):72-82. doi: 10.1016/j.vetimm.2014.09.008. Epub 2014 Oct 8. Vet Immunol Immunopathol. 2014. PMID: 25454082
-
Potent HIV-specific responses are enriched in a unique subset of CD8+ T cells that coexpresses CD4 on its surface.Blood. 2009 Oct 29;114(18):3841-53. doi: 10.1182/blood-2009-02-202481. Epub 2009 Aug 21. Blood. 2009. PMID: 19700667 Free PMC article.
-
CD4+ CD8+ double positive (DP) T cells in health and disease.Autoimmun Rev. 2004 Mar;3(3):215-20. doi: 10.1016/j.autrev.2003.09.001. Autoimmun Rev. 2004. PMID: 15110234 Review.
Cited by
-
BACH2-mediated CD28 and CD40LG axes contribute to pathogenesis and progression of T-cell lymphoblastic leukemia.Cell Death Dis. 2024 Jan 17;15(1):59. doi: 10.1038/s41419-024-06453-8. Cell Death Dis. 2024. PMID: 38233409 Free PMC article.
-
The role of immune cell signatures in the pathogenesis of ovarian-related diseases: a causal inference based on Mendelian randomization.Int J Surg. 2024 Oct 1;110(10):6541-6550. doi: 10.1097/JS9.0000000000001814. Int J Surg. 2024. PMID: 38884272 Free PMC article.
-
Living, Heat-Killed Limosilactobacillus mucosae and Its Cell-Free Supernatant Differentially Regulate Colonic Serotonin Receptors and Immune Response in Experimental Colitis.Nutrients. 2024 Feb 6;16(4):468. doi: 10.3390/nu16040468. Nutrients. 2024. PMID: 38398793 Free PMC article.
-
A nonhuman primate model for genital herpes simplex virus 2 infection that results in vaginal vesicular lesions, virus shedding, and seroconversion.PLoS Pathog. 2024 Sep 3;20(9):e1012477. doi: 10.1371/journal.ppat.1012477. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39226323 Free PMC article.
-
T Cell Peptide Prediction, Immune Response, and Host-Pathogen Relationship in Vaccinated and Recovered from Mild COVID-19 Subjects.Biomolecules. 2024 Sep 26;14(10):1217. doi: 10.3390/biom14101217. Biomolecules. 2024. PMID: 39456150 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

