The impact of rotavirus vaccination on acute diarrhea in Thai children under 5 years of age in the first year of universal implementation of rotavirus vaccines in the National Immunization Program (NIP) in Thailand: a 6-year analysis
- PMID: 37891542
- PMCID: PMC10604840
- DOI: 10.1186/s12889-023-16958-0
The impact of rotavirus vaccination on acute diarrhea in Thai children under 5 years of age in the first year of universal implementation of rotavirus vaccines in the National Immunization Program (NIP) in Thailand: a 6-year analysis
Abstract
Background: Two types of rotavirus vaccines (RVs), Rotarix (RV1) and RotaTeq (RV5), were licensed as optional vaccines in 2012 and became part of the National Immunization Program (NIP) in the fiscal year 2020 in Thailand. The main objective was to evaluate the impact of rotavirus vaccines on the burden of acute diarrheal severity ranging from outpatient visits, diarrheal-related admission or deaths in the pre-NIP period (fiscal year 2015-2019) and in the fiscal year 2020. The minor objectives were assessed on the monthly admission rate, rotavirus vaccine coverage rate and rotavirus vaccine completed dose (RotaC).
Methods: Data regarding OPD, admission, and death cases under the Thailand National Health Coverage (NHC) from fiscal year 2015-2020, which were recorded as International Classification of Diseases and Related Health Problem 10th (ICD-10), were analyzed.
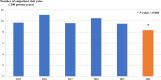
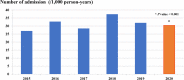
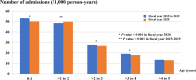
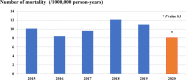
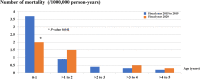
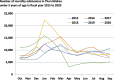
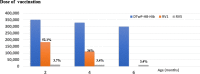
Results: The burden of diarrheal-related disease diminished after the rotavirus vaccine was introduced in the fiscal year 2020 when compared to the previous 5 fiscal years. The OPD visit rate decreased from 10.1 to 8.3 visits per 100 person-years (P < 0.001), or a 17.8% reduction (incidence rate ratio (IRR) = 0.82; 95% confidence interval (CI): 0.81 to 0.82). The admission rate significantly declined from 31.4 to 30.5 cases per 1,000 person-years, (P < 0.001), or a 2.9% reduction (IRR = 0.97; 95% CI: 0.96 to 0.98). The diarrheal-related mortality rate also subsided from 10.2 to 8.1 cases per 100,000 person-years (P 0.3), or a 20.0% reduction (IRR = 0.88; 95% CI: 0.50 to 1.22). The major population in both admissions and deaths was infants under 1 year of age (P < 0.001). Seasonality was seen as a constant bimodal pattern, with a significant decrease in monthly admissions after 6 months of rotavirus vaccine introduction to NIP (P < 0.001). RotaC was 37.4% in the first year of NIP.
Conclusions: The rotavirus vaccine had a potential benefit for reducing the diarrheal disease burden, especially in infants under one year of age. Seasonality outbreaks of acute diarrhea subsided after the rotavirus vaccine was introduced. The RotaC was fairly low in the first year of the NIP. The quality of the rotavirus vaccine should be warranted.
Trial registration: Number TCTR20220120003 , date of registration: 20/01/2022, site: Thai Clinical Trials Registry.
Keywords: Acute diarrhea; Children; Children under 5 years of age; Rotavirus; Rotavirus vaccine.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Vaccines for preventing rotavirus diarrhoea: vaccines in use.Cochrane Database Syst Rev. 2012 Nov 14;11:CD008521. doi: 10.1002/14651858.CD008521.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2019 Mar 25;3:CD008521. doi: 10.1002/14651858.CD008521.pub4 PMID: 23152260 Updated. Review.
-
Vaccines for preventing rotavirus diarrhoea: vaccines in use.Cochrane Database Syst Rev. 2012 Feb 15;(2):CD008521. doi: 10.1002/14651858.CD008521.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2012 Nov 14;11:CD008521. doi: 10.1002/14651858.CD008521.pub3 PMID: 22336845 Updated. Review.
-
Cost-effectiveness analysis of the implementation of a National Immunization Program for rotavirus vaccination in a country with a low rotavirus gastroenteritis-related mortality: A South Korean study.Vaccine. 2019 Aug 14;37(35):4987-4995. doi: 10.1016/j.vaccine.2019.07.030. Epub 2019 Jul 17. Vaccine. 2019. PMID: 31326252
-
Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia's National Childhood vaccine schedule.Pediatr Infect Dis J. 2011 Jan;30(1 Suppl):S25-9. doi: 10.1097/INF.0b013e3181fefdee. Pediatr Infect Dis J. 2011. PMID: 21183837
-
Early impact of rotavirus vaccination in children less than five years of age in Mozambique.Vaccine. 2018 Nov 12;36(47):7205-7209. doi: 10.1016/j.vaccine.2017.10.060. Epub 2017 Nov 8. Vaccine. 2018. PMID: 29128381
Cited by
-
Prevalence and risk factors associated with under-five years children diarrhea in Malawi: Application of survey logistic regression.Heliyon. 2024 Apr 6;10(7):e29335. doi: 10.1016/j.heliyon.2024.e29335. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38623245 Free PMC article.
-
A pilot study using hospital surveillance and a birth cohort to investigate enteric pathogens and malnutrition in children, Dili, Timor-Leste.PLoS One. 2024 Feb 1;19(2):e0296774. doi: 10.1371/journal.pone.0296774. eCollection 2024. PLoS One. 2024. PMID: 38300944 Free PMC article.
References
-
- Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–623. doi: 10.1016/S0140-6736(10)60755-6. - DOI - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

