Spaceflight Induces Strength Decline in Caenorhabditis elegans
- PMID: 37887314
- PMCID: PMC10605753
- DOI: 10.3390/cells12202470
Spaceflight Induces Strength Decline in Caenorhabditis elegans
Abstract
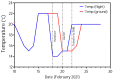
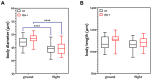
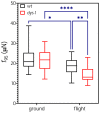
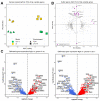
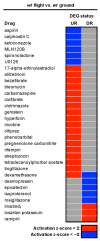
Background: Understanding and countering the well-established negative health consequences of spaceflight remains a primary challenge preventing safe deep space exploration. Targeted/personalized therapeutics are at the forefront of space medicine strategies, and cross-species molecular signatures now define the 'typical' spaceflight response. However, a lack of direct genotype-phenotype associations currently limits the robustness and, therefore, the therapeutic utility of putative mechanisms underpinning pathological changes in flight. Methods: We employed the worm Caenorhabditis elegans as a validated model of space biology, combined with 'NemaFlex-S' microfluidic devices for assessing animal strength production as one of the most reproducible physiological responses to spaceflight. Wild-type and dys-1 (BZ33) strains (a Duchenne muscular dystrophy (DMD) model for comparing predisposed muscle weak animals) were cultured on the International Space Station in chemically defined media before loading second-generation gravid adults into NemaFlex-S devices to assess individual animal strength. These same cultures were then frozen on orbit before returning to Earth for next-generation sequencing transcriptomic analysis. Results: Neuromuscular strength was lower in flight versus ground controls (16.6% decline, p < 0.05), with dys-1 significantly more (23% less strength, p < 0.01) affected than wild types. The transcriptional gene ontology signatures characterizing both strains of weaker animals in flight strongly corroborate previous results across species, enriched for upregulated stress response pathways and downregulated mitochondrial and cytoskeletal processes. Functional gene cluster analysis extended this to implicate decreased neuronal function, including abnormal calcium handling and acetylcholine signaling, in space-induced strength declines under the predicted control of UNC-89 and DAF-19 transcription factors. Finally, gene modules specifically altered in dys-1 animals in flight again cluster to neuronal/neuromuscular pathways, suggesting strength loss in DMD comprises a strong neuronal component that predisposes these animals to exacerbated strength loss in space. Conclusions: Highly reproducible gene signatures are strongly associated with space-induced neuromuscular strength loss across species and neuronal changes in calcium/acetylcholine signaling require further study. These results promote targeted medical efforts towards and provide an in vivo model for safely sending animals and people into deep space in the near future.
Keywords: C. elegans; International Space Station; astropharmacy; dystrophin; gene expression; microgravity; muscle atrophy; muscle strength; omics; spaceflight.
Conflict of interest statement
S.A.V. and M.R. are co-founders of NemaLife Inc., which has licensed the microfluidic technology for commercialization. T.A. is currently employed by NemaLife.
Figures

Similar articles
-
Microgravity alters the expressions of DNA repair genes and their regulatory miRNAs in space-flown Caenorhabditis elegans.Life Sci Space Res (Amst). 2023 May;37:25-38. doi: 10.1016/j.lssr.2023.02.002. Epub 2023 Feb 17. Life Sci Space Res (Amst). 2023. PMID: 37087176
-
Muscle strength deficiency and mitochondrial dysfunction in a muscular dystrophy model of Caenorhabditis elegans and its functional response to drugs.Dis Model Mech. 2018 Dec 4;11(12):dmm036137. doi: 10.1242/dmm.036137. Dis Model Mech. 2018. PMID: 30396907 Free PMC article.
-
The DNA damage response of C. elegans affected by gravity sensing and radiosensitivity during the Shenzhou-8 spaceflight.Mutat Res. 2017 Jan;795:15-26. doi: 10.1016/j.mrfmmm.2017.01.001. Epub 2017 Jan 7. Mutat Res. 2017. PMID: 28088539
-
Spaceflight bioreactor studies of cells and tissues.Adv Space Biol Med. 2002;8:177-95. doi: 10.1016/s1569-2574(02)08019-x. Adv Space Biol Med. 2002. PMID: 12951697 Review.
-
Caenorhabditis elegans in microgravity: An omics perspective.iScience. 2023 Jun 20;26(7):107189. doi: 10.1016/j.isci.2023.107189. eCollection 2023 Jul 21. iScience. 2023. PMID: 37456835 Free PMC article. Review.
Cited by
-
How to obtain an integrated picture of the molecular networks involved in adaptation to microgravity in different biological systems?NPJ Microgravity. 2024 May 1;10(1):50. doi: 10.1038/s41526-024-00395-3. NPJ Microgravity. 2024. PMID: 38693246 Free PMC article. Review.
-
Advancing insights into microgravity induced muscle changes using Caenorhabditis elegans as a model organism.NPJ Microgravity. 2024 Jul 26;10(1):79. doi: 10.1038/s41526-024-00418-z. NPJ Microgravity. 2024. PMID: 39060303 Free PMC article. Review.
References
-
- Hirayama J., Hattori A., Takahashi A., Furusawa Y., Tabuchi Y., Shibata M., Nagamatsu A., Yano S., Maruyama Y., Matsubara H., et al. Physiological Consequences of Space Flight, Including Abnormal Bone Metabolism, Space Radiation Injury, and Circadian Clock Dysregulation: Implications of Melatonin Use and Regulation as a Countermeasure. J. Pineal Res. 2023;74:e12834. doi: 10.1111/jpi.12834. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

