The master antioxidant defense is activated during EBV latent infection
- PMID: 37877721
- PMCID: PMC10688347
- DOI: 10.1128/jvi.00953-23
The master antioxidant defense is activated during EBV latent infection
Abstract
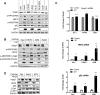
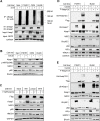
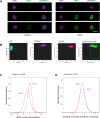
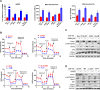
To our knowledge, this is the first report delineating the activation of the master antioxidant defense during EBV latency. We show that EBV-triggered reactive oxygen species production activates the Keap1-NRF2 pathway in EBV-transformed cells, and LMP1 plays a major role in this event, and the stress-related kinase TBK1 is required for NRF2 activation. Moreover, we show that the Keap1-NRF2 pathway is important for cell proliferation and EBV latency maintenance. Our findings disclose how EBV controls the balance between oxidative stress and antioxidant defense, which greatly improve our understanding of EBV latency and pathogenesis and may be leveraged to opportunities toward the improvement of therapeutic outcomes in EBV-associated diseases.
Keywords: EBV; Keap1; NRF2; antioxidant stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
p62-mediated Selective autophagy endows virus-transformed cells with insusceptibility to DNA damage under oxidative stress.PLoS Pathog. 2019 Apr 24;15(4):e1007541. doi: 10.1371/journal.ppat.1007541. eCollection 2019 Apr. PLoS Pathog. 2019. PMID: 31017975 Free PMC article.
-
Targeting Epstein-Barr virus oncoprotein LMP1-mediated high oxidative stress suppresses EBV lytic reactivation and sensitizes tumors to radiation therapy.Theranostics. 2020 Oct 25;10(26):11921-11937. doi: 10.7150/thno.46006. eCollection 2020. Theranostics. 2020. PMID: 33204320 Free PMC article.
-
LMP1-Induced Sumoylation Influences the Maintenance of Epstein-Barr Virus Latency through KAP1.J Virol. 2015 Aug;89(15):7465-77. doi: 10.1128/JVI.00711-15. Epub 2015 May 6. J Virol. 2015. PMID: 25948750 Free PMC article.
-
Management of COVID-19-induced cytokine storm by Keap1-Nrf2 system: a review.Inflammopharmacology. 2021 Oct;29(5):1347-1355. doi: 10.1007/s10787-021-00860-5. Epub 2021 Aug 9. Inflammopharmacology. 2021. PMID: 34373972 Free PMC article. Review.
-
The role of Nrf2 and PPARgamma in the improvement of oxidative stress in hypertension and cardiovascular diseases.Physiol Res. 2020 Dec 31;69(Suppl 4):S541-S553. doi: 10.33549/physiolres.934612. Physiol Res. 2020. PMID: 33656904 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

