The role of small extracellular vesicle-miRNAs in endometriosis
- PMID: 37877421
- PMCID: PMC10694411
- DOI: 10.1093/humrep/dead216
The role of small extracellular vesicle-miRNAs in endometriosis
Abstract
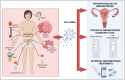
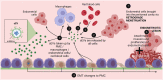
Endometriosis is defined by the presence of extrauterine endometrial-like tissue, which can cause pain and infertility in 10% of reproductive-age women. To date, the pathogenesis is poorly understood resulting in significant diagnostic delays and poor therapeutic outcomes in many women. Small extracellular vesicles (sEVs) (<200 nm) are cell-derived vesicles containing molecules that can influence gene expression and behaviour in target cells. One such cargo are microRNAs (miRNAs), which are short, non-coding RNAs mostly 19-25 nucleotides in length that regulate post-transcriptional gene expression. This mini-review focuses on the role of sEV-miRNAs, which are conceivably better biomarkers for endometriosis than free miRNAs, which reflect the true pathophysiological state in the body, as sEV-encapsulated miRNAs are protected from degradation compared to free miRNA and provide direct cell-to-cell communication via sEV surface proteins. sEV-miRNAs have been implicated in the immunomodulation of macrophages, the proliferation, migration and invasion of endometrial cells, and angiogenesis, all hallmarks of endometriosis. The diagnostic potential of sEV-miRNA was investigated in one study that reported the sensitivity and specificity of two sEV-miRNAs (hsa-miR-22-3p and hsa-miR-320a-3p) in distinguishing endometriosis from non-endometriosis cases. Only three studies have explored the therapeutic potential of sEV-miRNAs in vivo in mice-two looked into the role of sEV-hsa-miR-214-3p in decreasing fibrosis, and one investigated sEV-hsa-miR-30c-5p in suppressing the invasive and migratory potential of endometriotic lesions. While early results are encouraging, studies need to further address the potential influence of factors such as the menstrual cycle as well as the location and extent of endometriotic lesions on miRNA expression in sEVs. Given these findings, and extrapolating from other conditions such as cancer, diabetes, and pre-eclampsia, sEV-miRNAs could present an attractive and urgently needed future diagnostic and therapeutic target for millions of women suffering from endometriosis. However, research in this area is hampered by lack of adherence to the International Society for Extracellular Vesicles 2018 guideline in separating and characterising sEVs, as well as the World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project protocols.
Keywords: endometriosis; exosomal microRNAs; exosomes; miRNAs; sEV-miRNAs; small extracellular vesicles.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
None declared by all authors.
Figures


Similar articles
-
Serum outperforms plasma in small extracellular vesicle microRNA biomarker studies of adenocarcinoma of the esophagus.World J Gastroenterol. 2020 May 28;26(20):2570-2583. doi: 10.3748/wjg.v26.i20.2570. World J Gastroenterol. 2020. PMID: 32523312 Free PMC article.
-
Liver injury changes the biological characters of serum small extracellular vesicles and reprograms hepatic macrophages in mice.World J Gastroenterol. 2021 Nov 21;27(43):7509-7529. doi: 10.3748/wjg.v27.i43.7509. World J Gastroenterol. 2021. PMID: 34887646 Free PMC article.
-
Serum Exosomal MicroRNAs as Potential Circulating Biomarkers for Endometriosis.Dis Markers. 2020 Jan 23;2020:2456340. doi: 10.1155/2020/2456340. eCollection 2020. Dis Markers. 2020. PMID: 32076458 Free PMC article.
-
The miRNA Mirage: How Close Are We to Finding a Non-Invasive Diagnostic Biomarker in Endometriosis? A Systematic Review.Int J Mol Sci. 2018 Feb 17;19(2):599. doi: 10.3390/ijms19020599. Int J Mol Sci. 2018. PMID: 29463003 Free PMC article. Review.
-
Liquid Biopsy in Endometriosis: A Systematic Review.Int J Mol Sci. 2023 Mar 24;24(7):6116. doi: 10.3390/ijms24076116. Int J Mol Sci. 2023. PMID: 37047088 Free PMC article. Review.
Cited by
-
Diagnostic Value and Molecular Function of MicroRNAs in Endometrial Diseases: A Systematic Review.Cancers (Basel). 2024 Jun 30;16(13):2416. doi: 10.3390/cancers16132416. Cancers (Basel). 2024. PMID: 39001478 Free PMC article. Review.
-
Endometriosis: from iron and macrophages to exosomes. Is the sky clearing?Hum Reprod Open. 2024 May 30;2024(3):hoae034. doi: 10.1093/hropen/hoae034. eCollection 2024. Hum Reprod Open. 2024. PMID: 38905003 Free PMC article. No abstract available.
-
Saliva, a molecular reflection of the human body? Implications for diagnosis and treatment.Cell Stress. 2024 May 27;8:59-68. doi: 10.15698/cst2024.05.297. eCollection 2024. Cell Stress. 2024. PMID: 38826491 Free PMC article.
-
Extracellular vesicles and their content in the context of polycystic ovarian syndrome and endometriosis: a review.J Ovarian Res. 2024 Aug 5;17(1):160. doi: 10.1186/s13048-024-01480-7. J Ovarian Res. 2024. PMID: 39103867 Free PMC article. Review.
References
-
- Burney R. Fibrosis as a molecular hallmark of endometriosis pathophysiology. Fertil Steril 2022;118:203–204. - PubMed
-
- Canis M, Donnez J, Guzick D, Halme J, Rock J, Schenken R, Vernon M.. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997;67:817–821. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

