Lumped parameter liver simulation to predict acute haemodynamic alterations following partial resections
- PMID: 37876272
- PMCID: PMC10598422
- DOI: 10.1098/rsif.2023.0444
Lumped parameter liver simulation to predict acute haemodynamic alterations following partial resections
Abstract
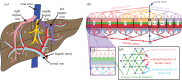
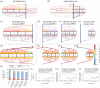
Partial liver resections are routinely performed in living donor liver transplantation and to debulk tumours in liver malignancies, but surgical decisions on vessel reconstruction for adequate inflow and outflow are challenging. Pre-operative evaluation is often limited to radiological imaging, which fails to account for post-resection haemodynamic alterations. Substantial evidence suggests post-surgical increase in local volume flow rate enhances shear stress, signalling hepatic regeneration, but excessive shear stress has been postulated to result in small for size syndrome and liver failure. Predicting haemodynamic alterations throughout the liver is particularly challenging due to the dendritic architecture of the vasculature, spanning several orders of magnitude in diameter. Therefore, we developed a mathematical lumped parameter model with realistic heterogeneities capturing inflow/outflow of the human liver to simulate acute perfusion alterations following surgical resection. Our model is parametrized using clinical measurements, relies on a single free parameter and accurately captures established perfusion characteristics. We quantify acute changes in volume flow rate, flow speed and wall shear stress following variable, realistic liver resections and make comparisons with the intact liver. Our numerical model runs in minutes and can be adapted to patient-specific anatomy, providing a novel computational tool aimed at assisting pre- and intra-operative surgical decisions for liver resections.
Keywords: haemodynamics; hepatic blood flow; liver resection; lumped parameter model.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Surgical procedures for decompression of excessive shear stress in small-for-size living donor liver transplantation--new hepatic vein reconstruction.Transplant Proc. 2005 Mar;37(2):1108-11. doi: 10.1016/j.transproceed.2004.11.029. Transplant Proc. 2005. PMID: 15848637
-
Partial hepatectomy hemodynamics changes: Experimental data explained by closed-loop lumped modeling.J Biomech. 2017 Jan 4;50:202-208. doi: 10.1016/j.jbiomech.2016.11.037. Epub 2016 Nov 16. J Biomech. 2017. PMID: 27890535
-
Laparoscopic living donor right hemihepatectomy with venous outflow reconstruction using cadaveric common iliac artery allograft: Case report and literature review.Medicine (Baltimore). 2017 Feb;96(7):e6167. doi: 10.1097/MD.0000000000006167. Medicine (Baltimore). 2017. PMID: 28207553 Free PMC article.
-
Hepatic vein in living donor liver transplantation.Hepatobiliary Pancreat Dis Int. 2020 Aug;19(4):318-323. doi: 10.1016/j.hbpd.2020.07.002. Epub 2020 Jul 10. Hepatobiliary Pancreat Dis Int. 2020. PMID: 32709407 Review.
-
WITHDRAWN: Methods of vascular occlusion for elective liver resections.Cochrane Database Syst Rev. 2009 Jan 21;2009(1):CD006409. doi: 10.1002/14651858.CD006409.pub3. Cochrane Database Syst Rev. 2009. PMID: 19160283 Free PMC article. Review.
References
-
- Lautt WW. 2009. Hepatic circulation: physiology and pathophysiology. In Colloquium series on integrated systems physiology: from molecule to function to disease (eds D Granger, JP Granger). San Rafael, CA: Morgan & Claypool Life Sciences. Copyright © 2010 by Morgan & Claypool Life Sciences.
-
- Couinaud C. 1992. The anatomy of the liver. Ann. Ital. Chir. 63, 693-697. - PubMed
-
- Dahmen U, Hall CA, Madrahimov N, Milekhin V, Dirsch O. 2007. Regulation of hepatic microcirculation in stepwise liver resection. Acta Gastroenterol. Belg. 70, 345-351. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

