Empagliflozin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial
- PMID: 37865101
- PMCID: PMC10957483
- DOI: 10.1016/S2213-8587(23)00253-X
Empagliflozin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial
Erratum in
-
Correction to Lancet Diabetes Endocrinol 2023; 11: 905-14.Lancet Diabetes Endocrinol. 2024 Jan;12(1):e1. doi: 10.1016/S2213-8587(23)00360-1. Epub 2023 Nov 28. Lancet Diabetes Endocrinol. 2024. PMID: 38040017 Free PMC article. No abstract available.
Abstract
Background: Empagliflozin has been proposed as a treatment for COVID-19 on the basis of its anti-inflammatory, metabolic, and haemodynamic effects. The RECOVERY trial aimed to assess its safety and efficacy in patients admitted to hospital with COVID-19.
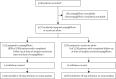
Methods: In the randomised, controlled, open-label RECOVERY trial, several possible treatments are compared with usual care in patients hospitalised with COVID-19. In this analysis, we assess eligible and consenting adults who were randomly allocated in a 1:1 ratio to either usual standard of care alone or usual standard of care plus oral empagliflozin 10 mg once daily for 28 days or until discharge (whichever came first) using web-based simple (unstratified) randomisation with allocation concealment. The primary outcome was 28-day mortality; secondary outcomes were duration of hospitalisation and (among participants not on invasive mechanical ventilation at baseline) the composite of invasive mechanical ventilation or death. On March 3, 2023 the independent data monitoring committee recommended that the investigators review the data and recruitment was consequently stopped on March 7, 2023. The ongoing RECOVERY trial is registered with ISRCTN (50189673) and ClinicalTrials.gov (NCT04381936).
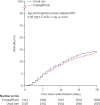
Findings: Between July 28, 2021 and March 6, 2023, 4271 patients were randomly allocated to receive either empagliflozin (2113 patients) or usual care alone (2158 patients). Primary and secondary outcome data were known for greater than 99% of randomly assigned patients. Overall, 289 (14%) of 2113 patients allocated to empagliflozin and 307 (14%) of 2158 patients allocated to usual care died within 28 days (rate ratio 0·96 [95% CI 0·82-1·13]; p=0·64). There was no evidence of significant differences in duration of hospitalisation (median 8 days for both groups) or the proportion of patients discharged from hospital alive within 28 days (1678 [79%] in the empagliflozin group vs 1677 [78%] in the usual care group; rate ratio 1·03 [95% CI 0·96-1·10]; p=0·44). Among those not on invasive mechanical ventilation at baseline, there was no evidence of a significant difference in the proportion meeting the composite endpoint of invasive mechanical ventilation or death (338 [16%] of 2084 vs 371 [17%] of 2143; risk ratio 0·95 [95% CI 0·84-1·08]; p=0·44). Two serious adverse events believed to be related to empagliflozin were reported: both were ketosis without acidosis.
Interpretation: In adults hospitalised with COVID-19, empagliflozin was not associated with reductions in 28-day mortality, duration of hospital stay, or risk of progressing to invasive mechanical ventilation or death so is not indicated for the treatment of such patients unless there is an established indication due to a different condition such as diabetes.
Funding: UK Research and Innovation (Medical Research Council) and National Institute of Health Research (MC_PC_19056), and Wellcome Trust (222406/Z/20/Z).
Translations: For the Nepali, Hindi, Indonesian (Bahasa) and Vietnamese translations of the abstract see Supplementary Materials section.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests NS, RH and MJL are named on grants to the University of Oxford from Boehringer Ingelheim for other research projects. All other authors declare no competing interests.
Figures

Comment in
-
Empagliflozin in adults hospitalised with COVID-19: a (null) hypothesis for RECOVERY.Lancet Diabetes Endocrinol. 2023 Dec;11(12):880-881. doi: 10.1016/S2213-8587(23)00263-2. Epub 2023 Oct 18. Lancet Diabetes Endocrinol. 2023. PMID: 37865100 No abstract available.
Similar articles
-
Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial.Lancet Respir Med. 2021 Dec;9(12):1419-1426. doi: 10.1016/S2213-2600(21)00435-5. Epub 2021 Oct 18. Lancet Respir Med. 2021. PMID: 34672950 Free PMC article. Clinical Trial.
-
Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial.Lancet. 2022 Jan 8;399(10320):143-151. doi: 10.1016/S0140-6736(21)01825-0. Epub 2021 Nov 17. Lancet. 2022. PMID: 34800427 Free PMC article. Clinical Trial.
-
Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial.Lancet. 2021 Feb 13;397(10274):605-612. doi: 10.1016/S0140-6736(21)00149-5. Epub 2021 Feb 2. Lancet. 2021. PMID: 33545096 Free PMC article. Clinical Trial.
-
Colchicine for the treatment of COVID-19.Cochrane Database Syst Rev. 2021 Oct 18;10(10):CD015045. doi: 10.1002/14651858.CD015045. Cochrane Database Syst Rev. 2021. PMID: 34658014 Free PMC article. Review.
-
Janus kinase inhibitors for the treatment of COVID-19.Cochrane Database Syst Rev. 2022 Jun 13;6(6):CD015209. doi: 10.1002/14651858.CD015209. Cochrane Database Syst Rev. 2022. PMID: 35695334 Free PMC article. Review.
Cited by
-
Immunity and Coagulation in COVID-19.Int J Mol Sci. 2024 Oct 19;25(20):11267. doi: 10.3390/ijms252011267. Int J Mol Sci. 2024. PMID: 39457048 Free PMC article. Review.
-
Adverse cardiovascular and kidney outcomes in people with SARS-CoV-2 treated with SGLT2 inhibitors.Commun Med (Lond). 2024 Sep 11;4(1):179. doi: 10.1038/s43856-024-00599-4. Commun Med (Lond). 2024. PMID: 39261630 Free PMC article.
-
Effect of sodium-glucose co-transporter-2 inhibitors on survival free of organ support in patients hospitalised for COVID-19 (ACTIV-4a): a pragmatic, multicentre, open-label, randomised, controlled, platform trial.Lancet Diabetes Endocrinol. 2024 Oct;12(10):725-734. doi: 10.1016/S2213-8587(24)00218-3. Epub 2024 Sep 6. Lancet Diabetes Endocrinol. 2024. PMID: 39250922 Clinical Trial.
-
Dapagliflozin for Critically Ill Patients With Acute Organ Dysfunction: The DEFENDER Randomized Clinical Trial.JAMA. 2024 Aug 6;332(5):401-411. doi: 10.1001/jama.2024.10510. JAMA. 2024. PMID: 38873723 Clinical Trial.
-
Potential use of sodium glucose co-transporter 2 inhibitors during acute illness: a systematic review based on COVID-19.Endocrine. 2024 Aug;85(2):660-675. doi: 10.1007/s12020-024-03758-8. Epub 2024 Mar 6. Endocrine. 2024. PMID: 38448675 Free PMC article.
References
-
- Nuffield Department of Population Health Renal Studies Group and the SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists' Consortium Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400:1788–1801. - PMC - PubMed
-
- Wang JH, Chen RD, Yang HK, et al. Inflammation-associated factors for predicting in-hospital mortality in patients with COVID-19. J Med Virol. 2021;93:2908–2917. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

