A Sexually Dimorphic Role for Intestinal Cannabinoid Receptor Subtype-1 in the Behavioral Expression of Anxiety
- PMID: 37862126
- PMCID: PMC10771877
- DOI: 10.1089/can.2023.0150
A Sexually Dimorphic Role for Intestinal Cannabinoid Receptor Subtype-1 in the Behavioral Expression of Anxiety
Abstract
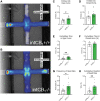
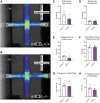
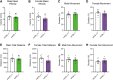
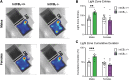
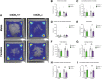
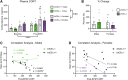
Background: Increasing evidence suggests that the endocannabinoid system (ECS) in the brain controls anxiety and may be a therapeutic target for the treatment of anxiety disorders. For example, both pharmacological and genetic disruption of cannabinoid receptor subtype-1 (CB1R) signaling in the central nervous system is associated with increased anxiety-like behaviors in rodents, while activating the system is anxiolytic. Sex is also a critical factor that controls the behavioral expression of anxiety; however, roles for the ECS in the gut in these processes and possible differences between sexes are largely unknown. Objective: In this study, we aimed to determine if CB1Rs in the intestinal epithelium exert control over anxiety-like behaviors in a sex-dependent manner. Methods: We subjected male and female mice with conditional deletion of CB1Rs in the intestinal epithelium (intCB1-/-) and controls (intCB1+/+) to the elevated plus maze (EPM), light/dark box, and open field test. Corticosterone (CORT) levels in plasma were measured at baseline and immediately after EPM exposure. Results: When compared with intCB1+/+ male mice, intCB1-/- male mice exhibited reduced levels of anxiety-like behaviors in the EPM and light/dark box. In contrast to male mice, no differences were found between female intCB1+/+ and intCB1-/- mice. Circulating CORT was higher in female versus male mice for both genotype groups at baseline and after EPM exposure; however, there was no effect of genotype on CORT levels. Conclusions: Collectively, these results indicate that genetic deletion of CB1Rs in the intestinal epithelium is associated with an anxiolytic phenotype in a sex-dependent manner.
Keywords: animal behavior; anxiety; cannabinoid receptors; intestinal epithelium; sex differences.
Conflict of interest statement
No competing financial interests exist.
Figures
Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Antioxidants for female subfertility.Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD007807. doi: 10.1002/14651858.CD007807.pub4. Cochrane Database Syst Rev. 2020. PMID: 32851663 Free PMC article.
-
Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article.
-
Comparison of cognitive behaviour therapy versus activity management, both delivered remotely, to treat paediatric chronic fatigue syndrome/myalgic encephalomyelitis: the UK FITNET-NHS RCT.Health Technol Assess. 2024 Oct;28(70):1-134. doi: 10.3310/VLRW6701. Health Technol Assess. 2024. PMID: 39485730 Free PMC article. Clinical Trial.
-
Antioxidants for female subfertility.Cochrane Database Syst Rev. 2017 Jul 28;7(7):CD007807. doi: 10.1002/14651858.CD007807.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Aug 27;8:CD007807. doi: 10.1002/14651858.CD007807.pub4. PMID: 28752910 Free PMC article. Updated. Review.
Cited by
-
Cannabinoids Block Fat-induced Incretin Release via CB1-dependent and CB1-independent Pathways in Intestinal Epithelium.Gastro Hep Adv. 2024 Jul 22;3(7):931-941. doi: 10.1016/j.gastha.2024.07.006. eCollection 2024. Gastro Hep Adv. 2024. PMID: 39318720 Free PMC article.

