How inhaled corticosteroids target inflammation in COPD
- PMID: 37852657
- PMCID: PMC10582931
- DOI: 10.1183/16000617.0084-2023
How inhaled corticosteroids target inflammation in COPD
Abstract
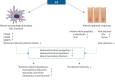
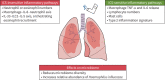
Inhaled corticosteroids (ICS) are the most commonly used anti-inflammatory drugs for the treatment of COPD. COPD has been previously described as a "corticosteroid-resistant" condition, but current clinical trial evidence shows that selected COPD patients, namely those with increased exacerbation risk plus higher blood eosinophil count (BEC), can benefit from ICS treatment. This review describes the components of inflammation modulated by ICS in COPD and the reasons for the variation in response to ICS between individuals. There are corticosteroid-insensitive inflammatory pathways in COPD, such as bacteria-induced macrophage interleukin-8 production and resultant neutrophil recruitment, but also corticosteroid-sensitive pathways including the reduction of type 2 markers and mast cell numbers. The review also describes the mechanisms whereby ICS can skew the lung microbiome, with reduced diversity and increased relative abundance, towards an excess of proteobacteria. BEC is a biomarker used to enable the selective use of ICS in COPD, but the clinical outcome in an individual is decided by a complex interacting network involving the microbiome and airway inflammation.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: D. Singh has received sponsorship to attend and speak at international meetings or honoraria for lecturing or attending advisory boards from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, Epiendo, Genentech, GlaxoSmithKline, Glenmark, Gossamerbio, Kinaset, Menarini, Novartis, Pulmatrix, Sanofi, Teva, Theravance and Verona. A. Beech, A. Higham and S. Lea have no conflicts of interest to declare.
Figures
Similar articles
-
The impact of inhaled corticosteroid and long-acting beta-agonist combination therapy on outcomes in COPD.Pulm Pharmacol Ther. 2008;21(3):540-50. doi: 10.1016/j.pupt.2007.12.004. Epub 2008 Jan 6. Pulm Pharmacol Ther. 2008. PMID: 18280761 Review.
-
Effectiveness using higher inhaled corticosteroid dosage in patients with COPD by different blood eosinophilic counts.Int J Chron Obstruct Pulmon Dis. 2016 Sep 21;11:2341-2348. doi: 10.2147/COPD.S115132. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27703344 Free PMC article. Clinical Trial.
-
Use of inhaled corticosteroids in COPD: improving efficacy.Expert Rev Respir Med. 2016;10(3):339-50. doi: 10.1586/17476348.2016.1151789. Expert Rev Respir Med. 2016. PMID: 26855301 Review.
-
Use of thiols and implications for the use of inhaled corticosteroids in the presence of oxidative stress in COPD.Respir Res. 2023 Jul 31;24(1):194. doi: 10.1186/s12931-023-02500-8. Respir Res. 2023. PMID: 37517999 Free PMC article. Review.
-
Blood eosinophil count, a marker of inhaled corticosteroid effectiveness in preventing COPD exacerbations in post-hoc RCT and observational studies: systematic review and meta-analysis.Respir Res. 2020 Jan 3;21(1):3. doi: 10.1186/s12931-019-1268-7. Respir Res. 2020. PMID: 31900184 Free PMC article.
Cited by
-
Eosinophils and therapeutic responses to steroids and biologics in COPD: a complex relationship.J Bras Pneumol. 2023 Dec 22;49(6):e20230360. doi: 10.36416/1806-3756/e20230360. J Bras Pneumol. 2023. PMID: 38126684 Free PMC article. No abstract available.
-
Analysis of Predictive Value of Cellular Inflammatory Factors and T Cell Subsets for Disease Recurrence and Prognosis in Patients with Acute Exacerbations of COPD.Int J Chron Obstruct Pulmon Dis. 2024 Nov 1;19:2361-2369. doi: 10.2147/COPD.S490152. eCollection 2024. Int J Chron Obstruct Pulmon Dis. 2024. PMID: 39502935 Free PMC article.
-
Inflammation mechanism and research progress of COPD.Front Immunol. 2024 Aug 9;15:1404615. doi: 10.3389/fimmu.2024.1404615. eCollection 2024. Front Immunol. 2024. PMID: 39185405 Free PMC article. Review.
-
Inhaled Corticosteroids in Subjects with Chronic Obstructive Pulmonary Disease: An Old, Unfinished History.Biomolecules. 2024 Feb 6;14(2):195. doi: 10.3390/biom14020195. Biomolecules. 2024. PMID: 38397432 Free PMC article. Review.
-
Transcriptional profiles of peripheral eosinophils in chronic obstructive pulmonary disease and asthma-An exploratory study.J Cell Mol Med. 2024 Oct;28(20):e70110. doi: 10.1111/jcmm.70110. J Cell Mol Med. 2024. PMID: 39422548 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
