The type-I interferon response potentiates seeded tau aggregation and exacerbates tau pathology
- PMID: 37849026
- PMCID: PMC10916982
- DOI: 10.1002/alz.13493
The type-I interferon response potentiates seeded tau aggregation and exacerbates tau pathology
Abstract
Introduction: Signatures of a type-I interferon (IFN-I) response are observed in the post mortem brain in Alzheimer's disease (AD) and other tauopathies. However, the effect of the IFN-I response on pathological tau accumulation remains unclear.
Methods: We examined the effects of IFN-I signaling in primary neural culture models of seeded tau aggregation and P301S-tau transgenic mouse models in the context of genetic deletion of the IFN-I receptor (IFNAR).
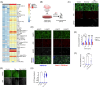
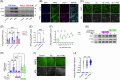
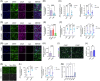
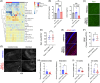
Results: Polyinosinic:polycytidylic acid (PolyI:C), a synthetic analog of viral nucleic acids, evoked a potent cytokine response that enhanced seeded aggregation of tau in an IFN-I-dependent manner. IFN-I-induced vulnerability could be pharmacologically prevented and was intrinsic to neurons. Aged P301S-tau mice lacking Ifnar1 had significantly reduced tau pathology compared to mice with intact IFN signaling.
Discussion: We identify a critical role for IFN-I in potentiating tau aggregation. IFN-I is therefore identified as a potential therapeutic target in AD and other tauopathies.
Highlights: Type-I IFN (IFN-I) promotes seeded tau aggregation in neural cultures. IFNAR inhibition prevents IFN-I driven sensitivity to tau aggregation. IFN-I driven vulnerability is intrinsic to neurons. Tau pathology is significantly reduced in aged P301S-tau mice lacking IFNAR.
Keywords: innate immunity; interferon; neuroinflammation; tau pathology; tauopathy.
© 2023 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors report no competing interests. Author disclosures are available in the supporting information.
Figures
Similar articles
-
The behavioural and neuropathologic sexual dimorphism and absence of MIP-3α in tau P301S mouse model of Alzheimer's disease.J Neuroinflammation. 2020 Feb 24;17(1):72. doi: 10.1186/s12974-020-01749-w. J Neuroinflammation. 2020. PMID: 32093751 Free PMC article.
-
ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy.Nature. 2017 Sep 28;549(7673):523-527. doi: 10.1038/nature24016. Epub 2017 Sep 20. Nature. 2017. PMID: 28959956 Free PMC article.
-
Neuronal Spleen tyrosine kinase (SYK) mediates cytokine release in Transgenic Tau P301S mice organotypic brain slice cultures.Neurosci Lett. 2020 Jun 11;729:134992. doi: 10.1016/j.neulet.2020.134992. Epub 2020 Apr 22. Neurosci Lett. 2020. PMID: 32334108
-
Cellular and pathological heterogeneity of primary tauopathies.Mol Neurodegener. 2021 Aug 23;16(1):57. doi: 10.1186/s13024-021-00476-x. Mol Neurodegener. 2021. PMID: 34425874 Free PMC article. Review.
-
Deletion of murine tau gene increases tau aggregation in a human mutant tau transgenic mouse model.Biochem Soc Trans. 2010 Aug;38(4):1001-5. doi: 10.1042/BST0381001. Biochem Soc Trans. 2010. PMID: 20658993 Review.
Cited by
-
Gut Microbiota as a Modifier of Huntington's Disease Pathogenesis.J Huntingtons Dis. 2024;13(2):133-147. doi: 10.3233/JHD-240012. J Huntingtons Dis. 2024. PMID: 38728199 Free PMC article. Review.
-
SARS-CoV-2-Induced Type I Interferon Signaling Dysregulation in Olfactory Networks Implications for Alzheimer's Disease.Curr Issues Mol Biol. 2024 May 10;46(5):4565-4579. doi: 10.3390/cimb46050277. Curr Issues Mol Biol. 2024. PMID: 38785545 Free PMC article.
-
Amyloid precursor protein induces reactive astrogliosis.bioRxiv [Preprint]. 2023 Dec 18:2023.12.18.571817. doi: 10.1101/2023.12.18.571817. bioRxiv. 2023. Update in: Acta Physiol (Oxf). 2024 Jun;240(6):e14142. doi: 10.1111/apha.14142 PMID: 38187544 Free PMC article. Updated. Preprint.
-
In sickness and in health-Type I interferon and the brain.Front Aging Neurosci. 2024 May 7;16:1403142. doi: 10.3389/fnagi.2024.1403142. eCollection 2024. Front Aging Neurosci. 2024. PMID: 38774266 Free PMC article. Review.
-
Type I interferon signaling, cognition and neurodegeneration following COVID-19: update on a mechanistic pathogenetic model with implications for Alzheimer's disease.Front Hum Neurosci. 2024 Mar 18;18:1352118. doi: 10.3389/fnhum.2024.1352118. eCollection 2024. Front Hum Neurosci. 2024. PMID: 38562226 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

