Molecular and phenotypic distinctions of macrophages in tolerant and susceptible to hypoxia rats
- PMID: 37842051
- PMCID: PMC10573310
- DOI: 10.7717/peerj.16052
Molecular and phenotypic distinctions of macrophages in tolerant and susceptible to hypoxia rats
Abstract
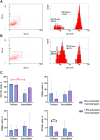
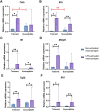
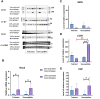
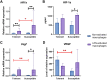
Individual hypoxia tolerance is a major influence on the course and outcome of infectious and inflammatory diseases. Macrophages, which play central roles in systemic inflammatory response and other immunity reactions, are subject to functional activation orchestrated by several transcription factors including hypoxia inducible factors (HIFs). HIF-1 expression levels and the lipopolysaccharide (LPS)-induced systemic inflammatory response severity have been shown to correlate with hypoxia tolerance. Molecular and functional features of macrophages, depending on the organisms resistance to hypoxia, can determine the severity of the course of infectious and inflammatory diseases, including the systemic inflammatory response. The purpose is the comparative molecular and functional characterization of non-activated and LPS-activated bone marrow-derived macrophages under normoxia in rats with different tolerance to oxygen deprivation. Hypoxia resistance was assessed by gasping time measurement in an 11,500 m altitude-equivalent hypobaric decompression chamber. Based on the outcome, the animals were assigned to three groups termed 'tolerant to hypoxia' (n = 12), 'normal', and 'susceptible to hypoxia' (n = 13). The 'normal' group was excluded from subsequent experiments. One month after hypoxia resistance test, the blood was collected from the tail vein to isolate monocytes. Non-activated and LPS-activated macrophage cultures were investigated by PCR, flow cytometry and Western blot methods. Gene expression patterns of non-activated cultured macrophages from tolerant and susceptible to hypoxia animals differed. We observed higher expression of VEGF and CD11b and lower expression of Tnfa, Il1b and Epas1 in non-activated cultures obtained from tolerant to hypoxia animals, whereas HIF-1α mRNA and protein expression levels were similar. LPS-activated macrophage cultures derived from susceptible to hypoxia animals expressed higher levels of Hif1a and CCR7 than the tolerant group; in addition, the activation was associated with increased content of HIF-1α in cell culture medium. The observed differences indicate a specific propensity toward pro-inflammatory macrophage polarization in susceptible to hypoxia rats.
Keywords: HIF-1; Hypoxia tolerance; Inflammation; Macrophage; Rats.
©2023 Dzhalilova et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures
Similar articles
-
Morphological and molecular-biological features of glioblastoma progression in tolerant and susceptible to hypoxia Wistar rats.Sci Rep. 2023 Aug 4;13(1):12694. doi: 10.1038/s41598-023-39914-9. Sci Rep. 2023. PMID: 37542119 Free PMC article.
-
Dependence of the severity of the systemic inflammatory response on resistance to hypoxia in male Wistar rats.J Inflamm Res. 2019 Mar 11;12:73-86. doi: 10.2147/JIR.S194581. eCollection 2019. J Inflamm Res. 2019. PMID: 30881082 Free PMC article.
-
MK8617 inhibits M1 macrophage polarization and inflammation via the HIF-1α/GYS1/UDPG/P2Y14 pathway.PeerJ. 2023 Jun 30;11:e15591. doi: 10.7717/peerj.15591. eCollection 2023. PeerJ. 2023. PMID: 37404479 Free PMC article.
-
Monocytes and dendritic cells in a hypoxic environment: Spotlights on chemotaxis and migration.Immunobiology. 2008;213(9-10):733-49. doi: 10.1016/j.imbio.2008.07.031. Epub 2008 Sep 21. Immunobiology. 2008. PMID: 18926289 Review.
-
Differences in Tolerance to Hypoxia: Physiological, Biochemical, and Molecular-Biological Characteristics.Biomedicines. 2020 Oct 18;8(10):428. doi: 10.3390/biomedicines8100428. Biomedicines. 2020. PMID: 33080959 Free PMC article. Review.
Cited by
-
Novel mechanisms of intestinal flora regulation in high-altitude hypoxia.Heliyon. 2024 Sep 20;10(20):e38220. doi: 10.1016/j.heliyon.2024.e38220. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39498080 Free PMC article.
-
Changes in the Expression of Genes Regulating the Response to Hypoxia, Inflammation, Cell Cycle, Apoptosis, and Epithelial Barrier Functioning during Colitis-Associated Colorectal Cancer Depend on Individual Hypoxia Tolerance.Int J Mol Sci. 2024 Jul 16;25(14):7801. doi: 10.3390/ijms25147801. Int J Mol Sci. 2024. PMID: 39063041 Free PMC article.
-
Molecular Mechanisms of Neuroprotection after the Intermittent Exposures of Hypercapnic Hypoxia.Int J Mol Sci. 2024 Mar 25;25(7):3665. doi: 10.3390/ijms25073665. Int J Mol Sci. 2024. PMID: 38612476 Free PMC article. Review.
-
Spontaneous and Stimulated Production of Cytokines by Blood Cells Ex Vivo as a Biomarker of Initially High or Low Hypoxia Resistance in Rats.Bull Exp Biol Med. 2024 Aug;177(4):418-422. doi: 10.1007/s10517-024-06200-1. Epub 2024 Sep 11. Bull Exp Biol Med. 2024. PMID: 39259469
References
-
- Al-Batran SE, Pauligk C, Wirtz R, Werner D, Steinmetz K, Homann N, Schmalenberg H, Hofheinz RD, Hartmann JT, Atmaca A, Altmannsberger HM, Jäger E. The validation of matrix metalloproteinase-9 mRNA gene expression as a predictor of outcome in patients with metastatic gastric cancer. Annals of Oncology. 2012;23(7):1699–1705. doi: 10.1093/annonc/mdr552. - DOI - PubMed
-
- Ardi VC, Van den Steen PE, Opdenakker G, Schweighofer B, Deryugina EI, Quigley JP. Neutrophil MMP-9 proenzyme, unencumbered by TIMP-1, undergoes efficient activation in vivo and catalytically induces angiogenesis via a basic fibroblast growth factor (FGF-2)/FGFR-2 pathway. Journal of Biological Chemistry. 2009;284(38):25854–25866. doi: 10.1074/jbc.M109.033472. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

