Mutations in genes related to myocyte contraction and ventricular septum development in non-syndromic tetralogy of Fallot
- PMID: 37840956
- PMCID: PMC10569225
- DOI: 10.3389/fcvm.2023.1249605
Mutations in genes related to myocyte contraction and ventricular septum development in non-syndromic tetralogy of Fallot
Abstract
Objective: Eighty percent of patients with a diagnosis of tetralogy of Fallot (TOF) do not have a known genetic etiology or syndrome. We sought to identify key molecular pathways and biological processes that are enriched in non-syndromic TOF, the most common form of cyanotic congenital heart disease, rather than single driver genes to elucidate the pathogenesis of this disease.
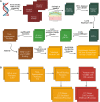
Methods: We undertook exome sequencing of 362 probands with non-syndromic TOF and their parents within the Pediatric Cardiac Genomics Consortium (PCGC). We identified rare (minor allele frequency <1 × 10-4), de novo variants to ascertain pathways and processes affected in this population to better understand TOF pathogenesis. Pathways and biological processes enriched in the PCGC TOF cohort were compared to 317 controls without heart defects (and their parents) from the Simons Foundation Autism Research Initiative (SFARI).
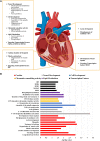
Results: A total of 120 variants in 117 genes were identified as most likely to be deleterious, with CHD7, CLUH, UNC13C, and WASHC5 identified in two probands each. Gene ontology analyses of these variants using multiple bioinformatic tools demonstrated significant enrichment in processes including cell cycle progression, chromatin remodeling, myocyte contraction and calcium transport, and development of the ventricular septum and ventricle. There was also a significant enrichment of target genes of SOX9, which is critical in second heart field development and whose loss results in membranous ventricular septal defects related to disruption of the proximal outlet septum. None of these processes was significantly enriched in the SFARI control cohort.
Conclusion: Innate molecular defects in cardiac progenitor cells and genes related to their viability and contractile function appear central to non-syndromic TOF pathogenesis. Future research utilizing our results is likely to have significant implications in stratification of TOF patients and delivery of personalized clinical care.
Keywords: congenital heart defect; conotruncal defects; de novo variants; exome sequencing; tetralogy of fallot.
© 2023 Harvey, Verma, Sedaghat, Hjelm, Morton, Seidman and Kumar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Genetic insights into non-syndromic Tetralogy of Fallot.Front Physiol. 2022 Oct 6;13:1012665. doi: 10.3389/fphys.2022.1012665. eCollection 2022. Front Physiol. 2022. PMID: 36277185 Free PMC article. Review.
-
Whole Exome Sequencing Reveals the Major Genetic Contributors to Nonsyndromic Tetralogy of Fallot.Circ Res. 2019 Feb 15;124(4):553-563. doi: 10.1161/CIRCRESAHA.118.313250. Circ Res. 2019. PMID: 30582441 Free PMC article.
-
Clinical Genetic Risk Variants Inform a Functional Protein Interaction Network for Tetralogy of Fallot.Circ Genom Precis Med. 2021 Aug;14(4):e003410. doi: 10.1161/CIRCGEN.121.003410. Epub 2021 Jul 30. Circ Genom Precis Med. 2021. PMID: 34328347 Free PMC article. Clinical Trial.
-
Gene-environment regulatory circuits of right ventricular pathology in tetralogy of fallot.J Mol Med (Berl). 2019 Dec;97(12):1711-1722. doi: 10.1007/s00109-019-01857-y. Epub 2019 Dec 13. J Mol Med (Berl). 2019. PMID: 31834445 Free PMC article.
-
Human Genetics of Tetralogy of Fallot and Double-Outlet Right Ventricle.Adv Exp Med Biol. 2024;1441:629-644. doi: 10.1007/978-3-031-44087-8_36. Adv Exp Med Biol. 2024. PMID: 38884738 Review.
Cited by
-
iPSC-Derived Cardiomyocytes as a Disease Model to Understand the Biology of Congenital Heart Defects.Cells. 2024 Aug 26;13(17):1430. doi: 10.3390/cells13171430. Cells. 2024. PMID: 39273002 Free PMC article. Review.
References
-
- Zimmerman MS, Smith AGC, Sable CA, Echko MM, Wilner LB, Olsen HE, et al. Global, regional, and national burden of congenital heart disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Child Adolesc Health. (2020) 4(3):185–200. 10.1016/S2352-4642(19)30402-X - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

