Glucosamine Improves Non-Alcoholic Fatty Liver Disease Induced by High-Fat and High-Sugar Diet through Regulating Intestinal Barrier Function, Liver Inflammation, and Lipid Metabolism
- PMID: 37836761
- PMCID: PMC10574579
- DOI: 10.3390/molecules28196918
Glucosamine Improves Non-Alcoholic Fatty Liver Disease Induced by High-Fat and High-Sugar Diet through Regulating Intestinal Barrier Function, Liver Inflammation, and Lipid Metabolism
Abstract
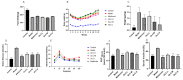
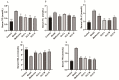
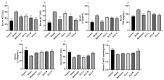
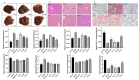
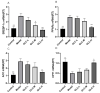
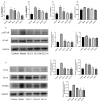
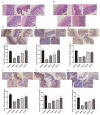
Non-alcoholic fatty liver disease (NAFLD) is a liver disease syndrome. The prevalence of NAFLD has continued to increase globally, and NAFLD has become a worldwide public health problem. Glucosamine (GLC) is an amino monosaccharide derivative of glucose. GLC has been proven to not only be effective in anti-inflammation applications, but also to modulate the gut microbiota effectively. Therefore, in this study, the therapeutic effect of GLC in the NAFLD context and the mechanisms underlying these effects were explored. Specifically, an NAFLD model was established by feeding mice a high-fat and high-sugar diet (HFHSD), and the HFHSD-fed NAFLD mice were treated with GLC. First, we investigated the effect of treating NAFLD mice with GLC by analyzing serum- and liver-related indicator levels. We found that GLC attenuated insulin resistance and inflammation, increased antioxidant function, and attenuated serum and liver lipid metabolism in the mice. Then, we investigated the mechanism underlying liver lipid metabolism, inflammation, and intestinal barrier function in these mice. We found that GLC can improve liver lipid metabolism and relieve insulin resistance and oxidative stress levels. In addition, GLC treatment increased intestinal barrier function, reduced LPS translocation, and reduced liver inflammation by inhibiting the activation of the LPS/TLR4/NF-κB pathway, thereby effectively ameliorating liver lesions in NAFLD mice.
Keywords: glucosamine; inflammation; intestinal barrier; liver lipid metabolism; non-alcoholic fatty liver disease.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
GOS Ameliorates Nonalcoholic Fatty Liver Disease Induced by High Fat and High Sugar Diet through Lipid Metabolism and Intestinal Microbes.Nutrients. 2022 Jul 1;14(13):2749. doi: 10.3390/nu14132749. Nutrients. 2022. PMID: 35807929 Free PMC article.
-
Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation.Free Radic Biol Med. 2017 Jan;102:188-202. doi: 10.1016/j.freeradbiomed.2016.11.037. Epub 2016 Nov 25. Free Radic Biol Med. 2017. PMID: 27890642
-
Lonicerae flos polysaccharides improve nonalcoholic fatty liver disease by activating the adenosine 5'-monophosphate-activated protein kinase pathway and reshaping gut microbiota.J Sci Food Agric. 2023 Dec;103(15):7721-7738. doi: 10.1002/jsfa.12854. Epub 2023 Jul 29. J Sci Food Agric. 2023. PMID: 37439182
-
Effects of curcumin on non-alcoholic fatty liver disease: A scientific metrogy study.Phytomedicine. 2024 Jan;123:155241. doi: 10.1016/j.phymed.2023.155241. Epub 2023 Nov 23. Phytomedicine. 2024. PMID: 38128395 Review.
-
Non-alcoholic fatty liver disease: the interplay between metabolism, microbes and immunity.Nat Metab. 2021 Dec;3(12):1596-1607. doi: 10.1038/s42255-021-00501-9. Epub 2021 Dec 20. Nat Metab. 2021. PMID: 34931080 Review.
Cited by
-
A new perspective in intestinal microecology: lifting the veil of exercise regulation of cardiometabolic diseases.Gut Microbes. 2024 Jan-Dec;16(1):2404141. doi: 10.1080/19490976.2024.2404141. Epub 2024 Sep 21. Gut Microbes. 2024. PMID: 39305272 Free PMC article. Review.
-
Glucosamine attenuates alcohol-induced acute liver injury via inhibiting oxidative stress and inflammation.Curr Res Food Sci. 2024 Feb 16;8:100699. doi: 10.1016/j.crfs.2024.100699. eCollection 2024. Curr Res Food Sci. 2024. PMID: 38420347 Free PMC article.
-
The effect of ertugliflozin in patients with nonalcoholic fatty liver disease associated with type 2 diabetes mellitus: A randomized controlled trial.Medicine (Baltimore). 2024 Nov 8;103(45):e40356. doi: 10.1097/MD.0000000000040356. Medicine (Baltimore). 2024. PMID: 39533572 Free PMC article. Clinical Trial.
-
The Interconnection between Hepatic Insulin Resistance and Metabolic Dysfunction-Associated Steatotic Liver Disease-The Transition from an Adipocentric to Liver-Centric Approach.Curr Issues Mol Biol. 2023 Nov 14;45(11):9084-9102. doi: 10.3390/cimb45110570. Curr Issues Mol Biol. 2023. PMID: 37998747 Free PMC article. Review.
References
-
- Duell P.B., Welty F.K., Miller M., Chait A., Hammond G., Ahmad Z., Cohen D.E., Horton J.D., Pressman G.S., Toth P.P. Nonalcoholic Fatty Liver Disease and Cardiovascular Risk: A Scientific Statement From the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2022;42:e168–e185. doi: 10.1161/ATV.0000000000000153. - DOI - PubMed
-
- Luo F., Smagris E., Martin S.A., Vale G., McDonald J.G., Fletcher J.A., Burgess S.C., Hobbs H.H., Cohen J.C. Hepatic TM6SF2 Is Required for Lipidation of VLDL in a Pre-Golgi Compartment in Mice and Rats. Cell Mol. Gastroenterol. Hepatol. 2022;13:879–899. doi: 10.1016/j.jcmgh.2021.12.008. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

