Shoot Organogenesis and Regeneration from Leaf Seedlings of Diospyros oleifera Cheng
- PMID: 37836247
- PMCID: PMC10574934
- DOI: 10.3390/plants12193507
Shoot Organogenesis and Regeneration from Leaf Seedlings of Diospyros oleifera Cheng
Abstract
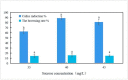
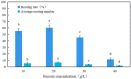
Persimmons (Diospyros) are economically important trees that are widely cultivated for wood production in China, and Diospyros oleifera Cheng is the main persimmon grafting stock. However, an efficient tissue culture system has not been perfected for D. oleifera due to the limits of proliferation and rooting cultures. Therefore, this study examined the effects of different plant growth regulators and concentrations on the primary culture of young embryos, induction of leaf callus, differentiation of adventitious shoots, and rooting culture of D. oleifera. The optimal formula for young embryo germination was 1/2 Murashige and Skoog (MS) medium containing 0.5 mg/L gibberellic acid (GA3); after 25 days, the sprouting rate of the young embryos was 67.3%. The best medium for leaf callus induction was 1/2MS medium containing 2.0 mg/L of 6-benzylaminopurine (6-BA) and 0.5 mg/L of naphthaleneacetic acid (NAA), and the callus induction rate was 88.9%. Then, the callus was transferred to 1/2MS medium containing 2.0 mg/L of zeatin (ZT), 0.5 mg/L of NAA, and 2.0 mg/L of thidiazuron (TDZ) to induce adventitious shoots; after 25 days, 5.4 buds were produced per explant, and the induction rate of the adventitious shoots was 88.3%. The adventitious shoots were transferred to 1/2MS medium containing 2.0 mg/L of ZT, 2.0 mg/L of 6-(γ,γ-dimethylallylamino)purine (2iP), and 0.1 mg/L of indole acetic acid (IAA) for the proliferation culture, for which the multiplication coefficient approached 7.5. After multiplication, the adventitious shoots were inoculated into 1/2MS medium containing 1.0 mg/L of indole butyric acid (IBA), 0.5 mg/L of NAA, and 1.0 mg/L of kinetin (KT); the rooting rate was 60.2%, and the average number of roots was 6.9.
Keywords: Callus induction; adventitious shoots; disinfection; rooting; tissue culture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Indirect shoot organogenesis from leaf explants of Adhatoda vasica Nees.Springerplus. 2014 Nov 3;3:648. doi: 10.1186/2193-1801-3-648. eCollection 2014. Springerplus. 2014. PMID: 25485191 Free PMC article.
-
Optimization of High-Efficiency Tissue Culture Regeneration Systems in Gray Poplar.Life (Basel). 2023 Sep 11;13(9):1896. doi: 10.3390/life13091896. Life (Basel). 2023. PMID: 37763300 Free PMC article.
-
Plant regeneration via somatic embryogenesis and shoot organogenesis from immature cotyledons of Camellia nitidissima Chi.J Plant Physiol. 2013 Sep 1;170(13):1202-11. doi: 10.1016/j.jplph.2013.03.019. Epub 2013 Jun 20. J Plant Physiol. 2013. PMID: 23790533
-
Gerbera micropropagation.Biotechnol Adv. 2013 Dec;31(8):1344-57. doi: 10.1016/j.biotechadv.2013.05.008. Epub 2013 Jun 4. Biotechnol Adv. 2013. PMID: 23743093 Review.
-
In vitro biotechnological advancements in Malabar nut (Adhatoda vasica Nees): Achievements, status and prospects.J Genet Eng Biotechnol. 2018 Dec;16(2):545-552. doi: 10.1016/j.jgeb.2018.03.007. Epub 2018 Mar 19. J Genet Eng Biotechnol. 2018. PMID: 30733772 Free PMC article. Review.
Cited by
-
Bulbil initiation: a comprehensive review on resources, development, and utilisation, with emphasis on molecular mechanisms, advanced technologies, and future prospects.Front Plant Sci. 2024 Apr 8;15:1343222. doi: 10.3389/fpls.2024.1343222. eCollection 2024. Front Plant Sci. 2024. PMID: 38650701 Free PMC article. Review.
-
Efficient plant regeneration through direct shoot organogenesis and two-step rooting in Eucommia ulmoides Oliver.Front Plant Sci. 2024 Sep 20;15:1444878. doi: 10.3389/fpls.2024.1444878. eCollection 2024. Front Plant Sci. 2024. PMID: 39372860 Free PMC article.
References
-
- Jiang Y., Cheng J.Y., Zhong X.H. Progress in persimmon Ti research. Mod. Gard. 2013;7:22–23. doi: 10.14051/j.cnki.xdyy.2013.07.049. - DOI
-
- Lin J.F., Lin H.T., Xie L.H., Lin Q.Y., Chen S.H., Zhao Y.F. Chemical composition, pharmacology, clinical application and development and utilization of persimmon leaves. Food Ferment. Ind. 2005;7:90–96. doi: 10.13995/j.cnki.11-1802/ts.2005.07.024. - DOI
-
- Xie Q.X., Zhuang D.H., Wu Y.H. Culture of Diospyros oleifera Cheng hypocotyl regeneration plants. Fruit Tree J. 2007;2:157–161+255.
-
- Fu J., Liang J., Wuyun T.N., Liang Y., Sun P., Li F. Sequence Analysis of ITS Regions and nd hA Gene for Determining Phylogenetic Relationship of Diospyros kaki (persimmon) With Other Related Wild Diospyros (Ebenaceae) Species. Plant Res. 2015;35:515–520.
Grants and funding
LinkOut - more resources
Full Text Sources

