Oncolytic Viral Therapy for Glioma by Recombinant Sindbis Virus
- PMID: 37835433
- PMCID: PMC10571546
- DOI: 10.3390/cancers15194738
Oncolytic Viral Therapy for Glioma by Recombinant Sindbis Virus
Abstract
Background: The characteristics of glioblastoma, such as drug resistance during treatment, short patient survival, and high recurrence rates, have made patients with glioblastoma more likely to benefit from oncolytic therapy.
Methods: In this study, we investigated the safety of the sindbis virus by injecting virus intravenously and intracranially in mice and evaluated the therapeutic effect of the virus carrying different combinations of IL-12, IL-7, and GM-CSF on glioma in a glioma-bearing mouse model.
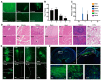
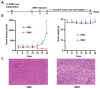
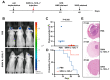
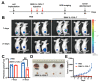
Results: SINV was autologously eliminated from the serum and organs as well as from neural networks after entering mice. Furthermore, SINV was restricted to the injection site in the tree shrew brain and did not spread throughout the whole brain. In addition, we found that SINV-induced apoptosis in conjunction with the stimulation of the immune system by tumor-killing cytokines substantially suppressed tumor development. It is worth mentioning that SINV carrying IL-7 and IL-12 had the most notable glioma-killing effect. Furthermore, in an intracranial glioma model, SINV containing IL-7 and IL-12 effectively prolonged the survival time of mice and inhibited glioma progression.
Conclusions: These results suggest that SINV has a significant safety profile as an oncolytic virus and that combining SINV with cytokines is an efficient treatment option for malignant gliomas.
Keywords: cytokines; glioblastoma; killing glioma; oncolytic virus; sindbis virus.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Oncolytic Activity of Sindbis Virus with the Help of GM-CSF in Hepatocellular Carcinoma.Int J Mol Sci. 2024 Jun 29;25(13):7195. doi: 10.3390/ijms25137195. Int J Mol Sci. 2024. PMID: 39000311 Free PMC article.
-
Oncolytic viral therapy for neuroblastoma cells with Sindbis virus AR339 strain.Pediatr Surg Int. 2015 Dec;31(12):1151-9. doi: 10.1007/s00383-015-3784-y. Epub 2015 Aug 23. Pediatr Surg Int. 2015. PMID: 26298056
-
PKR-NF-κB Pathway Upstream of IFN-β Induction Is Dysregulated in Oncolytic Sindbis Virus-infected HeLa Cells.Anticancer Res. 2023 Jul;43(7):2923-2932. doi: 10.21873/anticanres.16463. Anticancer Res. 2023. PMID: 37351990
-
Gospel of malignant Glioma: Oncolytic virus therapy.Gene. 2022 Apr 15;818:146217. doi: 10.1016/j.gene.2022.146217. Epub 2022 Jan 28. Gene. 2022. PMID: 35093451 Review.
-
Oncolytic Viruses for Malignant Glioma: On the Verge of Success?Viruses. 2021 Jul 2;13(7):1294. doi: 10.3390/v13071294. Viruses. 2021. PMID: 34372501 Free PMC article. Review.
Cited by
-
Oncolytic Activity of Sindbis Virus with the Help of GM-CSF in Hepatocellular Carcinoma.Int J Mol Sci. 2024 Jun 29;25(13):7195. doi: 10.3390/ijms25137195. Int J Mol Sci. 2024. PMID: 39000311 Free PMC article.
-
An Update on the Clinical Status, Challenges, and Future Directions of Oncolytic Virotherapy for Malignant Gliomas.Curr Treat Options Oncol. 2024 Jul;25(7):952-991. doi: 10.1007/s11864-024-01211-6. Epub 2024 Jun 19. Curr Treat Options Oncol. 2024. PMID: 38896326 Review.
-
Sindbis Virus Vaccine Platform: A Promising Oncolytic Virus-Mediated Approach for Ovarian Cancer Treatment.Int J Mol Sci. 2024 Mar 2;25(5):2925. doi: 10.3390/ijms25052925. Int J Mol Sci. 2024. PMID: 38474178 Free PMC article. Review.
References
-
- Young P.R., Ng L.F., Hall R.A., Smith D.W., Johansen C.A. Arbovirus infection. In: Farrar J., Hotez P.J., Junghanss T., Kang G., Lalloo D., White N.J., editors. Manson’s Tropical Diseases. 23rd ed. Saunders Ltd.; London, UK: 2013. pp. 129–161.
Grants and funding
- JCYJ20220818100801002/Shenzhen Fundamental Research Program
- ZDSYS20200811142401005/Shenzhen Key Laboratory of Viral Vectors for Biomedicine, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences
- 2020ZDB26/Key Laboratory of Quality Control Technology for Virus-Based Therapeutics, Guangdong Provincial Medical Products Administration
- 2022ZDZ13/Guangdong Provincial Medical Products Administration
- E1G023/The SIAT Innovation Program for Excellent Young Researchers
LinkOut - more resources
Full Text Sources
Other Literature Sources

