New Facets of DNA Double Strand Break Repair: Radiation Dose as Key Determinant of HR versus c-NHEJ Engagement
- PMID: 37834403
- PMCID: PMC10573367
- DOI: 10.3390/ijms241914956
New Facets of DNA Double Strand Break Repair: Radiation Dose as Key Determinant of HR versus c-NHEJ Engagement
Abstract
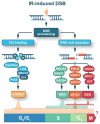
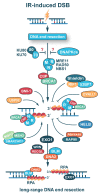
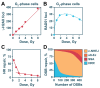
Radiation therapy is an essential component of present-day cancer management, utilizing ionizing radiation (IR) of different modalities to mitigate cancer progression. IR functions by generating ionizations in cells that induce a plethora of DNA lesions. The most detrimental among them are the DNA double strand breaks (DSBs). In the course of evolution, cells of higher eukaryotes have evolved four major DSB repair pathways: classical non-homologous end joining (c-NHEJ), homologous recombination (HR), alternative end-joining (alt-EJ), and single strand annealing (SSA). These mechanistically distinct repair pathways have different cell cycle- and homology-dependencies but, surprisingly, they operate with widely different fidelity and kinetics and therefore contribute unequally to cell survival and genome maintenance. It is therefore reasonable to anticipate tight regulation and coordination in the engagement of these DSB repair pathway to achieve the maximum possible genomic stability. Here, we provide a state-of-the-art review of the accumulated knowledge on the molecular mechanisms underpinning these repair pathways, with emphasis on c-NHEJ and HR. We discuss factors and processes that have recently come to the fore. We outline mechanisms steering DSB repair pathway choice throughout the cell cycle, and highlight the critical role of DNA end resection in this process. Most importantly, however, we point out the strong preference for HR at low DSB loads, and thus low IR doses, for cells irradiated in the G2-phase of the cell cycle. We further explore the molecular underpinnings of transitions from high fidelity to low fidelity error-prone repair pathways and analyze the coordination and consequences of this transition on cell viability and genomic stability. Finally, we elaborate on how these advances may help in the development of improved cancer treatment protocols in radiation therapy.
Keywords: DNA double strand breaks (DSBs); RAD51; cancer therapy; homologous recombination (HR); ionizing radiation (IR); radiation therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Analysis of chromatid-break-repair detects a homologous recombination to non-homologous end-joining switch with increasing load of DNA double-strand breaks.Mutat Res Genet Toxicol Environ Mutagen. 2021 Jul;867:503372. doi: 10.1016/j.mrgentox.2021.503372. Epub 2021 Jun 12. Mutat Res Genet Toxicol Environ Mutagen. 2021. PMID: 34266628
-
Chromosome breaks generated by low doses of ionizing radiation in G2-phase are processed exclusively by gene conversion.DNA Repair (Amst). 2020 May;89:102828. doi: 10.1016/j.dnarep.2020.102828. Epub 2020 Feb 27. DNA Repair (Amst). 2020. PMID: 32143127
-
Increased Gene Targeting in Hyper-Recombinogenic LymphoBlastoid Cell Lines Leaves Unchanged DSB Processing by Homologous Recombination.Int J Mol Sci. 2022 Aug 16;23(16):9180. doi: 10.3390/ijms23169180. Int J Mol Sci. 2022. PMID: 36012445 Free PMC article.
-
Alternative end-joining repair pathways are the ultimate backup for abrogated classical non-homologous end-joining and homologous recombination repair: Implications for the formation of chromosome translocations.Mutat Res Genet Toxicol Environ Mutagen. 2015 Nov;793:166-75. doi: 10.1016/j.mrgentox.2015.07.001. Epub 2015 Jul 4. Mutat Res Genet Toxicol Environ Mutagen. 2015. PMID: 26520387 Review.
-
Repair Pathway Choices and Consequences at the Double-Strand Break.Trends Cell Biol. 2016 Jan;26(1):52-64. doi: 10.1016/j.tcb.2015.07.009. Epub 2015 Oct 1. Trends Cell Biol. 2016. PMID: 26437586 Free PMC article. Review.
Cited by
-
Polθ: emerging synthetic lethal partner in homologous recombination-deficient tumors.Cancer Gene Ther. 2024 Nov;31(11):1619-1631. doi: 10.1038/s41417-024-00815-2. Epub 2024 Aug 9. Cancer Gene Ther. 2024. PMID: 39122831 Free PMC article. Review.
-
Self-Penetrating Oligonucleotide Derivatives: Features of Self-Assembly and Interactions with Serum and Intracellular Proteins.Pharmaceutics. 2023 Dec 14;15(12):2779. doi: 10.3390/pharmaceutics15122779. Pharmaceutics. 2023. PMID: 38140119 Free PMC article.
-
CDC20 Holds Novel Regulation Mechanism in RPA1 during Different Stages of DNA Damage to Induce Radio-Chemoresistance.Int J Mol Sci. 2024 Aug 1;25(15):8383. doi: 10.3390/ijms25158383. Int J Mol Sci. 2024. PMID: 39125953 Free PMC article.
-
Correctly identifying the cells of origin is essential for tailoring treatment and understanding the emergence of cancer stem cells and late metastases.Front Oncol. 2024 Apr 10;14:1369907. doi: 10.3389/fonc.2024.1369907. eCollection 2024. Front Oncol. 2024. PMID: 38660133 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

