Sex-Dependent Synaptic Remodeling of the Somatosensory Cortex in Mice With Prenatal Methadone Exposure
- PMID: 37829495
- PMCID: PMC10569410
- DOI: 10.3389/adar.2022.10400
Sex-Dependent Synaptic Remodeling of the Somatosensory Cortex in Mice With Prenatal Methadone Exposure
Abstract
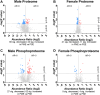
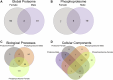
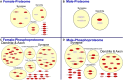
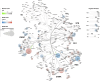
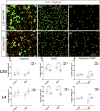
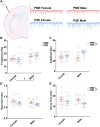
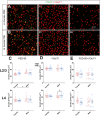
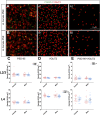
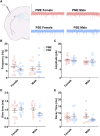
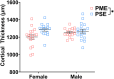
Rising opioid use among pregnant women has led to a growing population of neonates exposed to opioids during the prenatal period, but how opioids affect the developing brain remains to be fully understood. Animal models of prenatal opioid exposure have discovered deficits in somatosensory behavioral development that persist into adolescence suggesting opioid exposure induces long lasting neuroadaptations on somatosensory circuitry such as the primary somatosensory cortex (S1). Using a mouse model of prenatal methadone exposure (PME) that displays delays in somatosensory milestone development, we performed an un-biased multi-omics analysis and investigated synaptic functioning in the primary somatosensory cortex (S1), where touch and pain sensory inputs are received in the brain, of early adolescent PME offspring. PME was associated with numerous changes in protein and phosphopeptide abundances that differed considerably between sexes in the S1. Although prominent sex effects were discovered in the multi-omics assessment, functional enrichment analyses revealed the protein and phosphopeptide differences were associated with synapse-related cellular components and synaptic signaling-related biological processes, regardless of sex. Immunohistochemical analysis identified diminished GABAergic synapses in both layer 2/3 and 4 of PME offspring. These immunohistochemical and proteomic alterations were associated with functional consequences as layer 2/3 pyramidal neurons revealed reduced amplitudes and a lengthened decay constant of inhibitory postsynaptic currents. Lastly, in addition to reduced cortical thickness of the S1, cell-type marker analysis revealed reduced microglia density in the upper layer of the S1 that was primarily driven by PME females. Taken together, our studies show the lasting changes on synaptic function and microglia in S1 cortex caused by PME in a sex-dependent manner.
Keywords: methadone; neurodevelopment; prenatal opioid exposure; proteomics; somatosensory cortex.
Conflict of interest statement
CONFLICT OF INTEREST The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Prenatal methadone exposure selectively alters protein expression in primary motor cortex: Implications for synaptic function.Front Pharmacol. 2023 Feb 1;14:1124108. doi: 10.3389/fphar.2023.1124108. eCollection 2023. Front Pharmacol. 2023. PMID: 36817148 Free PMC article.
-
Perinatal Fentanyl Exposure Leads to Long-Lasting Impairments in Somatosensory Circuit Function and Behavior.J Neurosci. 2021 Apr 14;41(15):3400-3417. doi: 10.1523/JNEUROSCI.2470-20.2020. J Neurosci. 2021. PMID: 33853934 Free PMC article.
-
Prenatal Opioid Exposure Impairs Endocannabinoid and Glutamate Transmission in the Dorsal Striatum.eNeuro. 2022 Apr 20;9(2):ENEURO.0119-22.2022. doi: 10.1523/ENEURO.0119-22.2022. Print 2022 Mar-Apr. eNeuro. 2022. PMID: 35396255 Free PMC article.
-
Multielectrode Recordings in the Somatosensory System.In: Nicolelis MAL, editor. Methods for Neural Ensemble Recordings. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2008. Chapter 6. In: Nicolelis MAL, editor. Methods for Neural Ensemble Recordings. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2008. Chapter 6. PMID: 21204443 Free Books & Documents. Review.
-
[Research progress on barrel cortex and its plasticity].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2011 May;40(3):332-7. doi: 10.3785/j.issn.1008-9292.2011.03.018. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2011. PMID: 21671497 Review. Chinese.
Cited by
-
Early-life maturation of the somatosensory cortex: sensory experience and beyond.Front Neural Circuits. 2024 Jul 8;18:1430783. doi: 10.3389/fncir.2024.1430783. eCollection 2024. Front Neural Circuits. 2024. PMID: 39040685 Free PMC article. Review.
-
Prenatal methadone exposure selectively alters protein expression in primary motor cortex: Implications for synaptic function.Front Pharmacol. 2023 Feb 1;14:1124108. doi: 10.3389/fphar.2023.1124108. eCollection 2023. Front Pharmacol. 2023. PMID: 36817148 Free PMC article.
-
Alterations of brain microstructures in a mouse model of prenatal opioid exposure detected by diffusion MRI.Sci Rep. 2022 Oct 12;12(1):17085. doi: 10.1038/s41598-022-21416-9. Sci Rep. 2022. PMID: 36224335 Free PMC article.
-
Advances in animal models of prenatal opioid exposure.Trends Neurosci. 2024 May;47(5):367-382. doi: 10.1016/j.tins.2024.03.005. Epub 2024 Apr 12. Trends Neurosci. 2024. PMID: 38614891 Review.
-
Effects of prenatal opioid exposure on synaptic adaptations and behaviors across development.Neuropharmacology. 2023 Jan 1;222:109312. doi: 10.1016/j.neuropharm.2022.109312. Epub 2022 Nov 2. Neuropharmacology. 2023. PMID: 36334764 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

