Unlocking the role of non-coding RNAs in prostate cancer progression: exploring the interplay with the Wnt signaling pathway
- PMID: 37829301
- PMCID: PMC10565042
- DOI: 10.3389/fphar.2023.1269233
Unlocking the role of non-coding RNAs in prostate cancer progression: exploring the interplay with the Wnt signaling pathway
Abstract
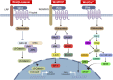
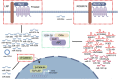
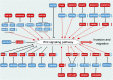
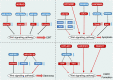
Prostate cancer (PCa) is one of the most common cancers in males, exhibiting a wide spectrum of clinical manifestations that pose challenges in its diagnosis and treatment. The Wnt signaling pathway, a conserved and complex pathway, is crucial for embryonic development, tissue homeostasis, and various physiological processes. Apart from the classical Wnt/β-catenin signaling pathway, there exist multiple non-classical Wnt signaling pathways, including the Wnt/PCP and Wnt/Ca2+ pathways. Non-coding RNAs (ncRNAs) are involved in the occurrence and development of PCa and the response to PCa treatment. ncRNAs are known to execute diverse regulatory roles in cellular processes, despite their inability to encode proteins. Among them, microRNAs, long non-coding RNAs, and circular RNAs play key roles in the regulation of the Wnt signaling pathway in PCa. Aberrant expression of these ncRNAs and dysregulation of the Wnt signaling pathway are one of the causes of cell proliferation, apoptosis, invasion, migration, and angiogenesis in PCa. Moreover, these ncRNAs affect the characteristics of PCa cells and hold promise as diagnostic and prognostic biomarkers. Herein, we summarize the role of ncRNAs in the regulation of the Wnt signaling pathway during the development of PCa. Additionally, we present an overview of the current progress in research on the correlation between these molecules and clinical features of the disease to provide novel insights and strategies for the treatment of PCa.
Keywords: Wnt signaling pathway; circRNA; lncRNA; miRNA; non-coding RNA; prostate cancer.
Copyright © 2023 Bu, Li and Tian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The emerging role of non-coding RNAs in the Wnt/β-catenin signaling pathway in Prostate Cancer.Pathol Res Pract. 2024 Feb;254:155134. doi: 10.1016/j.prp.2024.155134. Epub 2024 Jan 14. Pathol Res Pract. 2024. PMID: 38277746 Review.
-
Deciphering the Enigmatic Influence: Non-Coding RNAs Orchestrating Wnt/β-Catenin Signaling Pathway in Tumor Progression.Int J Mol Sci. 2023 Sep 10;24(18):13909. doi: 10.3390/ijms241813909. Int J Mol Sci. 2023. PMID: 37762212 Free PMC article. Review.
-
A comprehensive insight into the correlation between ncRNAs and the Wnt/β-catenin signalling pathway in gastric cancer pathogenesis.Cell Commun Signal. 2023 Jun 29;21(1):166. doi: 10.1186/s12964-023-01092-6. Cell Commun Signal. 2023. PMID: 37386429 Free PMC article. Review.
-
A spotlight on the interplay between Wnt/β-catenin signaling and circular RNAs in hepatocellular carcinoma progression.Front Oncol. 2023 Jul 21;13:1224138. doi: 10.3389/fonc.2023.1224138. eCollection 2023. Front Oncol. 2023. PMID: 37546393 Free PMC article. Review.
-
Current landscape of exosomal non-coding RNAs in prostate cancer: Modulators and biomarkers.Noncoding RNA Res. 2024 Jul 20;9(4):1351-1362. doi: 10.1016/j.ncrna.2024.07.003. eCollection 2024 Dec. Noncoding RNA Res. 2024. PMID: 39247145 Free PMC article. Review.
Cited by
-
Heme Oxygenase-1 and Prostate Cancer: Function, Regulation, and Implication in Cancer Therapy.Int J Mol Sci. 2024 Aug 24;25(17):9195. doi: 10.3390/ijms25179195. Int J Mol Sci. 2024. PMID: 39273143 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

