Field-friendly anti-PGL-I serosurvey in children to monitor Mycobacterium leprae transmission in Bihar, India
- PMID: 37828950
- PMCID: PMC10565223
- DOI: 10.3389/fmed.2023.1260375
Field-friendly anti-PGL-I serosurvey in children to monitor Mycobacterium leprae transmission in Bihar, India
Abstract
Background: It has been amply described that levels of IgM antibodies against Mycobacterium leprae (M. leprae) phenolic glycolipid I (PGL-I) correlate strongly with the bacterial load in an infected individual. These findings have generated the concept of using seropositivity for antibodies against M. leprae PGL-I as an indicator of the proportion of the population that has been infected. Although anti-PGL-I IgM levels provide information on whether an individual has ever been infected, their presence cannot discriminate between recent and past infections. Since infection in (young) children by definition indicates recent transmission, we piloted the feasibility of assessment of anti-PGL-I IgM seroprevalence among children in a leprosy endemic area in India as a proxy for recent M. leprae transmission.
Material and methods: A serosurvey for anti-PGL-I IgM antibodies among children in highly leprosy endemic villages in Bihar, India, was performed, applying the quantitative anti-PGL-I UCP-LFA cassette combined with low-invasive, small-volume fingerstick blood (FSB).
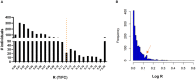
Results: Local staff obtained FSB of 1,857 children (age 3-11 years) living in 12 leprosy endemic villages in Bihar; of these, 215 children (11.58%) were seropositive for anti-PGL-I IgM.
Conclusion: The anti-PGL-I seroprevalence level of 11.58% among children corresponds with the seroprevalence levels described in studies in other leprosy endemic areas over the past decades where no prophylactic interventions have taken place. The anti-PGL-I UCP-LFA was found to be a low-complexity tool that could be practically combined with serosurveys and was well-accepted by both healthcare staff and the population. On route to leprosy elimination, quantitative anti-PGL-I serology in young children holds promise as a strategy to monitor recent M. leprae transmission in an area.
Keywords: anti-M. leprae PGL-I antibodies; children; diagnostics; infection; leprosy; serosurvey; transmission; upconversion.
Copyright © 2023 Pierneef, Malaviya, van Hooij, Sundar, Singh, Kumar, de Jong, Meuldijk, Kumar, Zhou, Cloots, Corstjens, Hasker and Geluk.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Detection and Monitoring of Mycobacterium leprae Infection in Nine Banded Armadillos (Dasypus novemcinctus) Using a Quantitative Rapid Test.Front Microbiol. 2021 Oct 28;12:763289. doi: 10.3389/fmicb.2021.763289. eCollection 2021. Front Microbiol. 2021. PMID: 34777319 Free PMC article.
-
Detection of anti-M. leprae antibodies in children in leprosy-endemic areas: A systematic review.PLoS Negl Trop Dis. 2021 Aug 27;15(8):e0009667. doi: 10.1371/journal.pntd.0009667. eCollection 2021 Aug. PLoS Negl Trop Dis. 2021. PMID: 34449763 Free PMC article.
-
Fingerstick test quantifying humoral and cellular biomarkers indicative for M. leprae infection.Clin Biochem. 2019 Apr;66:76-82. doi: 10.1016/j.clinbiochem.2019.01.007. Epub 2019 Jan 26. Clin Biochem. 2019. PMID: 30695682
-
The role of Mycobacterium leprae phenolic glycolipid I (PGL-I) in serodiagnosis and in the pathogenesis of leprosy.Lepr Rev. 2011 Dec;82(4):344-57. Lepr Rev. 2011. PMID: 22439275 Review.
-
Recent Laboratory Advances in Diagnostics and Monitoring Response to Treatment in Leprosy.Indian Dermatol Online J. 2019 Mar-Apr;10(2):106-114. doi: 10.4103/idoj.IDOJ_260_18. Indian Dermatol Online J. 2019. PMID: 30984583 Free PMC article. Review.
Cited by
-
Molecular and Serological Surveillance for Mycobacterium leprae and Mycobacterium lepromatosis in Wild Red Squirrels (Sciurus vulgaris) from Scotland and Northern England.Animals (Basel). 2024 Jul 7;14(13):2005. doi: 10.3390/ani14132005. Animals (Basel). 2024. PMID: 38998117 Free PMC article.
-
Self-healing in leprosy: A systematic review.PLoS Negl Trop Dis. 2024 Sep 12;18(9):e0012434. doi: 10.1371/journal.pntd.0012434. eCollection 2024 Sep. PLoS Negl Trop Dis. 2024. PMID: 39264863 Free PMC article.
-
Target product profiles: leprosy diagnostics.Bull World Health Organ. 2024 Apr 1;102(4):288-295. doi: 10.2471/BLT.23.290881. Epub 2024 Feb 22. Bull World Health Organ. 2024. PMID: 38562197 Free PMC article.
References
-
- World Health Organization . Leprosy. Available online at: https://www.who.int/news-room/fact-sheets/detail/leprosy (accessed August 18, 2023).
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

