Farnesoid X receptor: From Structure to Function and Its Pharmacology in Liver Fibrosis
- PMID: 37815898
- PMCID: PMC11272191
- DOI: 10.14336/AD.2023.0830
Farnesoid X receptor: From Structure to Function and Its Pharmacology in Liver Fibrosis
Abstract
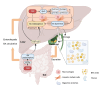
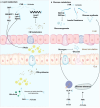
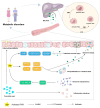
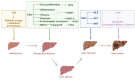
The farnesoid X receptor (FXR), a ligand-activated transcription factor, plays a crucial role in regulating bile acid metabolism within the enterohepatic circulation. Beyond its involvement in metabolic disorders and immune imbalances affecting various tissues, FXR is implicated in microbiota modulation, gut-to-brain communication, and liver disease. The liver, as a pivotal metabolic and detoxification organ, is susceptible to damage from factors such as alcohol, viruses, drugs, and high-fat diets. Chronic or recurrent liver injury can culminate in liver fibrosis, which, if left untreated, may progress to cirrhosis and even liver cancer, posing significant health risks. However, therapeutic options for liver fibrosis remain limited in terms of FDA-approved drugs. Recent insights into the structure of FXR, coupled with animal and clinical investigations, have shed light on its potential pharmacological role in hepatic fibrosis. Progress has been achieved in both fundamental research and clinical applications. This review critically examines recent advancements in FXR research, highlighting challenges and potential mechanisms underlying its role in liver fibrosis treatment.
Conflict of interest statement
The authors declare that there is no competition of interest.
Figures


Similar articles
-
The role of bile acids in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis.Mol Aspects Med. 2017 Aug;56:34-44. doi: 10.1016/j.mam.2017.04.004. Epub 2017 May 5. Mol Aspects Med. 2017. PMID: 28442273 Free PMC article. Review.
-
The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer.Nat Rev Gastroenterol Hepatol. 2021 May;18(5):335-347. doi: 10.1038/s41575-020-00404-2. Epub 2021 Feb 10. Nat Rev Gastroenterol Hepatol. 2021. PMID: 33568795 Review.
-
Recent insights into farnesoid X receptor in non-alcoholic fatty liver disease.World J Gastroenterol. 2014 Oct 7;20(37):13493-500. doi: 10.3748/wjg.v20.i37.13493. World J Gastroenterol. 2014. PMID: 25309079 Free PMC article. Review.
-
FXR signaling in the enterohepatic system.Mol Cell Endocrinol. 2013 Apr 10;368(1-2):17-29. doi: 10.1016/j.mce.2012.05.004. Epub 2012 May 17. Mol Cell Endocrinol. 2013. PMID: 22609541 Free PMC article. Review.
-
Exploring the role of a novel postbiotic bile acid: Interplay with gut microbiota, modulation of the farnesoid X receptor, and prospects for clinical translation.Microbiol Res. 2024 Oct;287:127865. doi: 10.1016/j.micres.2024.127865. Epub 2024 Aug 5. Microbiol Res. 2024. PMID: 39121702 Review.
References
-
- Yan N, Yan T, Xia Y, Hao H, Wang G, Gonzalez FJ (2021). The pathophysiological function of non-gastrointestinal farnesoid X receptor. Pharmacol Ther, 226:107867. - PubMed
-
- Fang Y, Hegazy L, Finck BN, Elgendy B (2021). Recent Advances in the Medicinal Chemistry of Farnesoid X Receptor. J Med Chem, 64:17545-17571. - PubMed
-
- Anakk S, Dean AE (2020). Fxr-alpha Skips Alternatively in Liver Metabolism. Gastroenterology, 159:1655-1657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
