The PTP1B Inhibitor Trodusquemine (MSI-1436) Improves Glucose Uptake in Equine Metabolic Syndrome Affected Liver through Anti-Inflammatory and Antifibrotic Activity
- PMID: 37808009
- PMCID: PMC10560121
- DOI: 10.1155/2023/3803056
The PTP1B Inhibitor Trodusquemine (MSI-1436) Improves Glucose Uptake in Equine Metabolic Syndrome Affected Liver through Anti-Inflammatory and Antifibrotic Activity
Abstract
Background: Hyperactivation of protein tyrosine phosphatase (PTP1B) has been associated with several metabolic malfunctions ranging from insulin resistance, metaflammation, lipotoxicity, and hyperglycaemia. Liver metabolism failure has been proposed as a core element in underlying endocrine disorders through persistent inflammation and highly fibrotic phenotype.
Methods: In this study, the outcomes of PTP1B inhibition using trodusquemine (MSI-1436) on key equine metabolic syndrome (EMS)-related alterations including inflammation, fibrosis, and glucose uptake have been analyzed in liver explants collected from EMS-affected horses using various analytical techniques, namely, flow cytometry, RT-qPCR, and Western blot.
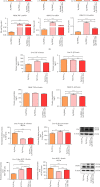
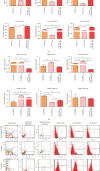
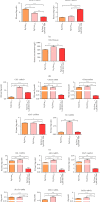
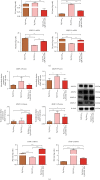
Results: PTP1B inhibition using trodusquemine resulted in decreased proinflammatory cytokines (IL-1β, TNF-α, and IL-6) release from liver and PBMC affected by EMS and regulated expression of major proinflammatory microRNAs such as miR-802 and miR-211. Moreover, MSI-1436 enhanced the anti-inflammatory profile of livers by elevating the expression of IL-10 and IL-4 and activating CD4+CD25+Foxp3+ regulatory T cells in treated PBMC. Similarly, the inhibitor attenuated fibrogenic pathways in the liver by downregulating TGF-β/NOX1/4 axis and associated MMP-2/9 overactivation. Interestingly, PTP1B inhibition ameliorated the expression of TIMP-1 and Smad7, both important antifibrotic mediators. Furthermore, application of MSI-1436 was found to augment the abundance of glycosylated Glut-2, which subsequently expanded the glucose absorption in the EMS liver, probably due to an enhanced Glut-2 stability and half-life onto the plasma cell membranes.
Conclusion: Taken together, the presented data suggest that the PTP1B inhibition strategy and the use of its specific inhibitor MSI-1436 represents a promising option for the improvement of liver tissue integrity and homeostasis in the course of EMS and adds more insights for ongoing clinical trials for human MetS management.
Copyright © 2023 Lynda Bourebaba et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
The PTP1B inhibitor MSI-1436 ameliorates liver insulin sensitivity by modulating autophagy, ER stress and systemic inflammation in Equine metabolic syndrome affected horses.Front Endocrinol (Lausanne). 2023 Mar 20;14:1149610. doi: 10.3389/fendo.2023.1149610. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37020593 Free PMC article.
-
MSI-1436 improves EMS adipose derived progenitor stem cells in the course of adipogenic differentiation through modulation of ER stress, apoptosis, and oxidative stress.Stem Cell Res Ther. 2021 Feb 3;12(1):97. doi: 10.1186/s13287-020-02102-x. Stem Cell Res Ther. 2021. PMID: 33536069 Free PMC article.
-
Trodusquemine (MSI-1436) Restores Metabolic Flexibility and Mitochondrial Dynamics in Insulin-Resistant Equine Hepatic Progenitor Cells (HPCs).Cells. 2024 Jan 14;13(2):152. doi: 10.3390/cells13020152. Cells. 2024. PMID: 38247843 Free PMC article.
-
Can Allostery Be a Key Strategy for Targeting PTP1B in Drug Discovery? A Lesson from Trodusquemine.Int J Mol Sci. 2023 Jun 1;24(11):9621. doi: 10.3390/ijms24119621. Int J Mol Sci. 2023. PMID: 37298571 Free PMC article. Review.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
Cited by
-
From Tyrosine Kinases to Tyrosine Phosphatases: New Therapeutic Targets in Cancers and Beyond.Pharmaceutics. 2024 Jul 1;16(7):888. doi: 10.3390/pharmaceutics16070888. Pharmaceutics. 2024. PMID: 39065585 Free PMC article. Review.
-
The combination of a PTP1B inhibitor, TNFR2 blocker, and PD‑1 antibody suppresses the progression of non‑small cell lung cancer tumors by enhancing immunocompetence.Oncol Rep. 2024 Nov;52(5):149. doi: 10.3892/or.2024.8808. Epub 2024 Sep 20. Oncol Rep. 2024. PMID: 39301655 Free PMC article.
-
Early life interventions metformin and trodusquemine metabolically reprogram the developing mouse liver through transcriptomic alterations.Aging Cell. 2024 Sep;23(9):e14227. doi: 10.1111/acel.14227. Epub 2024 May 27. Aging Cell. 2024. PMID: 38798180 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

