WNT Signaling in Stem Cells: A Look into the Non-Canonical Pathway
- PMID: 37804416
- PMCID: PMC10799802
- DOI: 10.1007/s12015-023-10610-5
WNT Signaling in Stem Cells: A Look into the Non-Canonical Pathway
Abstract
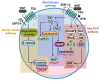
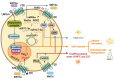
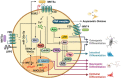
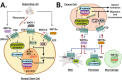
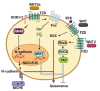
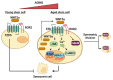
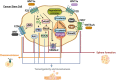
Tissue homeostasis is crucial for multicellular organisms, wherein the loss of cells is compensated by generating new cells with the capacity for proliferation and differentiation. At the origin of these populations are the stem cells, which have the potential to give rise to cells with both capabilities, and persevere for a long time through the self-renewal and quiescence. Since the discovery of stem cells, an enormous effort has been focused on learning about their functions and the molecular regulation behind them. Wnt signaling is widely recognized as essential for normal and cancer stem cell. Moreover, β-catenin-dependent Wnt pathway, referred to as canonical, has gained attention, while β-catenin-independent Wnt pathways, known as non-canonical, have remained conspicuously less explored. However, recent evidence about non-canonical Wnt pathways in stem cells begins to lay the foundations of a conceivably vast field, and on which we aim to explain this in the present review. In this regard, we addressed the different aspects in which non-canonical Wnt pathways impact the properties of stem cells, both under normal conditions and also under disease, specifically in cancer.
Keywords: Cancer; Cancer stem cell; Non-canonical Wnt; Stemness.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Frizzled7 Promotes Epithelial-to-mesenchymal Transition and Stemness Via Activating Canonical Wnt/β-catenin Pathway in Gastric Cancer.Int J Biol Sci. 2018 Feb 12;14(3):280-293. doi: 10.7150/ijbs.23756. eCollection 2018. Int J Biol Sci. 2018. PMID: 29559846 Free PMC article.
-
The Wnt/β-catenin pathway regulates self-renewal of cancer stem-like cells in human gastric cancer.Mol Med Rep. 2012 May;5(5):1191-6. doi: 10.3892/mmr.2012.802. Epub 2012 Feb 21. Mol Med Rep. 2012. PMID: 22367735
-
Drug discovery efforts toward inhibitors of canonical Wnt/β-catenin signaling pathway in the treatment of cancer: A composition-of-matter review (2010-2020).Drug Discov Today. 2022 Apr;27(4):1115-1127. doi: 10.1016/j.drudis.2021.11.014. Epub 2021 Nov 17. Drug Discov Today. 2022. PMID: 34800684 Review.
-
Wnt/beta-catenin signaling in cancer stemness and malignant behavior.Curr Opin Cell Biol. 2007 Apr;19(2):150-8. doi: 10.1016/j.ceb.2007.02.007. Epub 2007 Feb 16. Curr Opin Cell Biol. 2007. PMID: 17306971 Review.
-
FoxM1 and Wnt/β-catenin signaling in glioma stem cells.Cancer Res. 2012 Nov 15;72(22):5658-62. doi: 10.1158/0008-5472.CAN-12-0953. Epub 2012 Nov 8. Cancer Res. 2012. PMID: 23139209 Free PMC article. Review.
Cited by
-
Wnt/Ca2+ pathway inhibits neural differentiation of human dental pulp stem cells in vitro.J Dent Sci. 2024 Oct;19(4):2090-2099. doi: 10.1016/j.jds.2024.04.011. Epub 2024 Apr 24. J Dent Sci. 2024. PMID: 39347028 Free PMC article.
-
Epigenetic and Immune Mechanisms Linking Breastfeeding to Lower Breast Cancer Rates.Med Sci Monit. 2024 Nov 5;30:e945451. doi: 10.12659/MSM.945451. Med Sci Monit. 2024. PMID: 39497379 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

