Outcomes of hypothalamic oxytocin neuron-driven cardioprotection after acute myocardial infarction
- PMID: 37801130
- PMCID: PMC10558415
- DOI: 10.1007/s00395-023-01013-1
Outcomes of hypothalamic oxytocin neuron-driven cardioprotection after acute myocardial infarction
Abstract
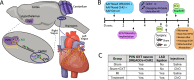
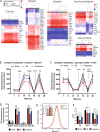
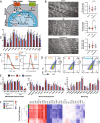
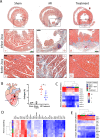
Altered autonomic balance is a hallmark of numerous cardiovascular diseases, including myocardial infarction (MI). Although device-based vagal stimulation is cardioprotective during chronic disease, a non-invasive approach to selectively stimulate the cardiac parasympathetic system immediately after an infarction does not exist and is desperately needed. Cardiac vagal neurons (CVNs) in the brainstem receive powerful excitation from a population of neurons in the paraventricular nucleus (PVN) of the hypothalamus that co-release oxytocin (OXT) and glutamate to excite CVNs. We tested if chemogenetic activation of PVN-OXT neurons following MI would be cardioprotective. The PVN of neonatal rats was transfected with vectors to selectively express DREADDs within OXT neurons. At 6 weeks of age, an MI was induced and DREADDs were activated with clozapine-N-oxide. Seven days following MI, patch-clamp electrophysiology confirmed the augmented excitatory neurotransmission from PVN-OXT neurons to downstream nuclei critical for parasympathetic activity with treatment (43.7 ± 10 vs 86.9 ± 9 pA; MI vs. treatment), resulting in stark improvements in survival (85% vs. 95%; MI vs. treatment), inflammation, fibrosis assessed by trichrome blue staining, mitochondrial function assessed by Seahorse assays, and reduced incidence of arrhythmias (50% vs. 10% cumulative incidence of ventricular fibrillation; MI vs. treatment). Myocardial transcriptomic analysis provided molecular insight into potential cardioprotective mechanisms, which revealed the preservation of beneficial signaling pathways, including muscarinic receptor activation, in treated animals. These comprehensive results demonstrate that the PVN-OXT network could be a promising therapeutic target to quickly activate beneficial parasympathetic-mediated cellular pathways within the heart during the early stages of infarction.
Keywords: Arrhythmia; Cardioprotection; Infarction; Mitochondria; Oxytocin; Parasympathetic nervous system.
© 2023. Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures



Similar articles
-
Chronic activation of hypothalamic oxytocin neurons improves cardiac function during left ventricular hypertrophy-induced heart failure.Cardiovasc Res. 2017 Sep 1;113(11):1318-1328. doi: 10.1093/cvr/cvx084. Cardiovasc Res. 2017. PMID: 28472396 Free PMC article.
-
Oxytocin neuron activation prevents hypertension that occurs with chronic intermittent hypoxia/hypercapnia in rats.Am J Physiol Heart Circ Physiol. 2016 Jun 1;310(11):H1549-57. doi: 10.1152/ajpheart.00808.2015. Epub 2016 Mar 25. Am J Physiol Heart Circ Physiol. 2016. PMID: 27016581 Free PMC article.
-
Sex Differences in the Hypothalamic Oxytocin Pathway to Locus Coeruleus and Augmented Attention with Chemogenetic Activation of Hypothalamic Oxytocin Neurons.Int J Mol Sci. 2021 Aug 7;22(16):8510. doi: 10.3390/ijms22168510. Int J Mol Sci. 2021. PMID: 34445224 Free PMC article.
-
The paraventricular nucleus and heart failure.Exp Physiol. 2014 Feb;99(2):332-9. doi: 10.1113/expphysiol.2013.072678. Epub 2013 Dec 6. Exp Physiol. 2014. PMID: 24317407 Review.
-
The interplay between glutamatergic circuits and oxytocin neurons in the hypothalamus and its relevance to neurodevelopmental disorders.J Neuroendocrinol. 2021 Dec;33(12):e13061. doi: 10.1111/jne.13061. Epub 2021 Nov 16. J Neuroendocrinol. 2021. PMID: 34786775 Free PMC article. Review.
Cited by
-
Semaglutide ameliorates cardiac remodeling in male mice by optimizing energy substrate utilization through the Creb5/NR4a1 axis.Nat Commun. 2024 Jun 4;15(1):4757. doi: 10.1038/s41467-024-48970-2. Nat Commun. 2024. PMID: 38834564 Free PMC article.
-
Sex differences in cardiac transcriptomic response to neonatal sleep apnea.Physiol Rep. 2024 Jul;12(13):e16110. doi: 10.14814/phy2.16110. Physiol Rep. 2024. PMID: 38981849 Free PMC article.
-
Molecular and cellular neurocardiology in heart disease.J Physiol. 2024 May 22:10.1113/JP284739. doi: 10.1113/JP284739. Online ahead of print. J Physiol. 2024. PMID: 38778747
References
-
- Abe M, Rastelli DD, Gomez AC, Cingolani E, Lee Y, Soni PR, Fishbein MC, Lehman TJA, Shimada K, Crother TR, Chen S, Noval Rivas M, Arditi M. IL-1-dependent electrophysiological changes and cardiac neural remodeling in a mouse model of Kawasaki disease vasculitis. Clin Exp Immunol. 2020;199:303–313. doi: 10.1111/cei.13401. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

