Diminishing neuronal acidification by channelrhodopsins with low proton conduction
- PMID: 37801078
- PMCID: PMC10558203
- DOI: 10.7554/eLife.86833
Diminishing neuronal acidification by channelrhodopsins with low proton conduction
Abstract
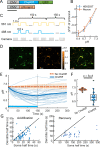
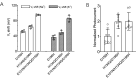
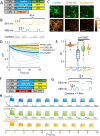
Many channelrhodopsins are permeable to protons. We found that in neurons, activation of a high-current channelrhodopsin, CheRiff, led to significant acidification, with faster acidification in the dendrites than in the soma. Experiments with patterned optogenetic stimulation in monolayers of HEK cells established that the acidification was due to proton transport through the opsin, rather than through other voltage-dependent channels. We identified and characterized two opsins which showed large photocurrents, but small proton permeability, PsCatCh2.0 and ChR2-3M. PsCatCh2.0 showed excellent response kinetics and was also spectrally compatible with simultaneous voltage imaging with QuasAr6a. Stimulation-evoked acidification is a possible source of disruptions to cell health in scientific and prospective therapeutic applications of optogenetics. Channelrhodopsins with low proton permeability are a promising strategy for avoiding these problems.
Keywords: acidification; electrophysiology; molecular biophysics; mouse; neuroscience; optogenetics; structural biology.
© 2023, Hayward et al.
Conflict of interest statement
RH, FB, SY, SG, AC No competing interests declared
Figures




Update of
-
Diminishing neuronal acidification by channelrhodopsins with low proton conduction.bioRxiv [Preprint]. 2023 Sep 14:2023.02.07.527404. doi: 10.1101/2023.02.07.527404. bioRxiv. 2023. Update in: Elife. 2023 Oct 06;12:RP86833. doi: 10.7554/eLife.86833 PMID: 36798192 Free PMC article. Updated. Preprint.
Similar articles
-
Diminishing neuronal acidification by channelrhodopsins with low proton conduction.bioRxiv [Preprint]. 2023 Sep 14:2023.02.07.527404. doi: 10.1101/2023.02.07.527404. bioRxiv. 2023. Update in: Elife. 2023 Oct 06;12:RP86833. doi: 10.7554/eLife.86833 PMID: 36798192 Free PMC article. Updated. Preprint.
-
Multidimensional screening yields channelrhodopsin variants having improved photocurrent and order-of-magnitude reductions in calcium and proton currents.J Biol Chem. 2019 Mar 15;294(11):3806-3821. doi: 10.1074/jbc.RA118.006996. Epub 2019 Jan 4. J Biol Chem. 2019. PMID: 30610117 Free PMC article.
-
Co-expressing fast channelrhodopsin with step-function opsin overcomes spike failure due to photocurrent desensitization in optogenetics: a theoretical study.J Neural Eng. 2022 Apr 6;19(2). doi: 10.1088/1741-2552/ac6061. J Neural Eng. 2022. PMID: 35320791
-
Channelrhodopsins: From Phototaxis to Optogenetics.Biochemistry (Mosc). 2023 Oct;88(10):1555-1570. doi: 10.1134/S0006297923100115. Biochemistry (Mosc). 2023. PMID: 38105024 Review.
-
Optogenetic Modulation of Ion Channels by Photoreceptive Proteins.Adv Exp Med Biol. 2021;1293:73-88. doi: 10.1007/978-981-15-8763-4_5. Adv Exp Med Biol. 2021. PMID: 33398808 Review.
Cited by
-
Behavioral control through the direct, focal silencing of neuronal activity.Cell Chem Biol. 2024 Jul 18;31(7):1324-1335.e20. doi: 10.1016/j.chembiol.2024.04.003. Epub 2024 May 9. Cell Chem Biol. 2024. PMID: 38729162
-
Metadynamics simulations reveal mechanisms of Na+ and Ca2+ transport in two open states of the channelrhodopsin chimera, C1C2.PLoS One. 2024 Sep 6;19(9):e0309553. doi: 10.1371/journal.pone.0309553. eCollection 2024. PLoS One. 2024. PMID: 39241014 Free PMC article.
References
-
- Adam Y, Kim JJ, Lou S, Zhao Y, Xie ME, Brinks D, Wu H, Mostajo-Radji MA, Kheifets S, Parot V, Chettih S, Williams KJ, Gmeiner B, Farhi SL, Madisen L, Buchanan EK, Kinsella I, Zhou D, Paninski L, Harvey CD, Zeng H, Arlotta P, Campbell RE, Cohen AE. Voltage imaging and optogenetics reveal behaviour-dependent changes in hippocampal dynamics. Nature. 2019;569:413–417. doi: 10.1038/s41586-019-1166-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

