Endothelial KIR2 channel dysfunction in aged cerebral parenchymal arterioles
- PMID: 37801044
- PMCID: PMC10907073
- DOI: 10.1152/ajpheart.00279.2023
Endothelial KIR2 channel dysfunction in aged cerebral parenchymal arterioles
Abstract
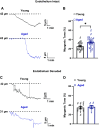
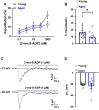
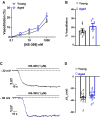
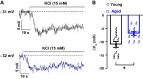
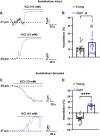
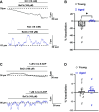
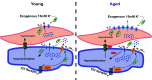
Aging is associated with cognitive decline via incompletely understood mechanisms. Cerebral microvascular dysfunction occurs in aging, particularly impaired endothelium-mediated dilation. Parenchymal arterioles are bottlenecks of the cerebral microcirculation, and dysfunction causes a mismatch in nutrient demand and delivery, leaving neurons at risk. Extracellular nucleotides elicit parenchymal arteriole dilation by activating endothelial purinergic receptors (P2Y), leading to opening of K+ channels, including inwardly-rectifying K+ channels (KIR2). These channels amplify hyperpolarizing signals, resulting in dilation. However, it remains unknown if endothelial P2Y and KIR2 signaling are altered in brain parenchymal arterioles during aging. We hypothesized that aging impairs endothelial P2Y and KIR2 function in parenchymal arterioles. We observed reduced dilation to the purinergic agonist 2-methyl-S-ADP (1 µM) in arterioles from Aged (>24-month-old) mice when compared to Young (4-6 months of age) despite similar hyperpolarization in endothelial cells tubes. No differences were observed in vasodilation or endothelial cell hyperpolarization to activation of small- and intermediate-conductance Ca2+-activated K+ channels (KCa2.3 / KCa3.1) by NS309. Hyperpolarization to 15 mM [K+]E was smaller in Aged than Young mice, despite a paradoxical increased dilation in Aged arterioles to 15 mM [K+]E that was unchanged by endothelium removal. KIR2 Inhibition attenuated vasodilatory responses to 15 mM [K+]E and 1 µM 2-me-S-ADP in both Young and Aged arterioles. Further, we observed a significant increase in myogenic tone in Aged parenchymal arterioles, which was not enhanced by endothelium removal. We conclude that aging impairs endothelial KIR2 channel function in the cerebral microcirculation with possible compensation by smooth muscle cells.
Keywords: aging; endothelium-dependent hyperpolarization; inwardly-rectifying K+ channel; parenchymal arterioles; purinergic receptors.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
Comment in
-
To err, KIR2 that is, on the side of vasodilation in aging.Am J Physiol Heart Circ Physiol. 2023 Dec 1;325(6):H1415-H1417. doi: 10.1152/ajpheart.00670.2023. Epub 2023 Oct 20. Am J Physiol Heart Circ Physiol. 2023. PMID: 37861649 No abstract available.
Similar articles
-
Comparison of cognitive behaviour therapy versus activity management, both delivered remotely, to treat paediatric chronic fatigue syndrome/myalgic encephalomyelitis: the UK FITNET-NHS RCT.Health Technol Assess. 2024 Oct;28(70):1-134. doi: 10.3310/VLRW6701. Health Technol Assess. 2024. PMID: 39485730 Free PMC article. Clinical Trial.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
A multicomponent psychosocial intervention to reduce substance use by adolescents involved in the criminal justice system: the RISKIT-CJS RCT.Public Health Res (Southampt). 2023 Mar;11(3):1-77. doi: 10.3310/FKPY6814. Public Health Res (Southampt). 2023. PMID: 37254608
-
Lamotrigine versus levetiracetam or zonisamide for focal epilepsy and valproate versus levetiracetam for generalised and unclassified epilepsy: two SANAD II non-inferiority RCTs.Health Technol Assess. 2021 Dec;25(75):1-134. doi: 10.3310/hta25750. Health Technol Assess. 2021. PMID: 34931602 Clinical Trial.
-
Topical fluoride as a cause of dental fluorosis in children.Cochrane Database Syst Rev. 2024 Jun 20;6(6):CD007693. doi: 10.1002/14651858.CD007693.pub3. Cochrane Database Syst Rev. 2024. PMID: 38899538 Review.
Cited by
-
Amyloid beta accumulation in TgF344-AD rats is associated with reduced cerebral capillary endothelial Kir2.1 expression and neurovascular uncoupling.Geroscience. 2023 Oct;45(5):2909-2926. doi: 10.1007/s11357-023-00841-2. Epub 2023 Jun 16. Geroscience. 2023. PMID: 37326915 Free PMC article.
-
Impairment of microvascular endothelial Kir2.1 channels contributes to endothelial dysfunction in human hypertension.Am J Physiol Heart Circ Physiol. 2024 Oct 1;327(4):H1004-H1015. doi: 10.1152/ajpheart.00732.2023. Epub 2024 Aug 30. Am J Physiol Heart Circ Physiol. 2024. PMID: 39212765
-
Functional, Structural and Proteomic Effects of Ageing in Resistance Arteries.Int J Mol Sci. 2024 Feb 23;25(5):2601. doi: 10.3390/ijms25052601. Int J Mol Sci. 2024. PMID: 38473847 Free PMC article. Review.
-
Ebbs and tides of endothelial K+ currents that regulate blood flow.Am J Physiol Heart Circ Physiol. 2024 Nov 1;327(5):H1205-H1207. doi: 10.1152/ajpheart.00678.2024. Epub 2024 Oct 11. Am J Physiol Heart Circ Physiol. 2024. PMID: 39392476 No abstract available.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

