Vagal Nerve Stimulation Reduces Ventricular Arrhythmias and Mitigates Adverse Neural Cardiac Remodeling Post-Myocardial Infarction
- PMID: 37791302
- PMCID: PMC10543930
- DOI: 10.1016/j.jacbts.2023.03.025
Vagal Nerve Stimulation Reduces Ventricular Arrhythmias and Mitigates Adverse Neural Cardiac Remodeling Post-Myocardial Infarction
Abstract
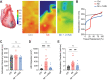
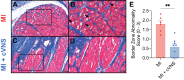
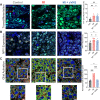
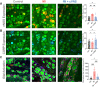
This study sought to evaluate the impact of chronic vagal nerve stimulation (cVNS) on cardiac and extracardiac neural structure/function after myocardial infarction (MI). Groups were control, MI, and MI + cVNS; cVNS was started 2 days post-MI. Terminal experiments were performed 6 weeks post-MI. MI impaired left ventricular mechanical function, evoked anisotropic electrical conduction, increased susceptibility to ventricular tachycardia and fibrillation, and altered neuronal and glial phenotypes in the stellate and dorsal root ganglia, including glial activation. cVNS improved cardiac mechanical function and reduced ventricular tachycardia/ventricular fibrillation post-MI, partly by stabilizing activation/repolarization in the border zone. MI-associated extracardiac neural remodeling, particularly glial activation, was mitigated with cVNS.
Keywords: myocardial infarction; neurocardiology; sympathetic nervous system; vagal nerve stimulation; ventricular tachycardia/ventricular fibrillation.
© 2023 The Authors.
Conflict of interest statement
This work was supported by the National Institutes of Health through the Office of the Director Grant OT2 OD023848 (Drs Shivkumar, Ardell, Ajijola, and Hoover), the National Institute of Biomedical Imaging and Bioengineering Grant U01 EB025138 (Drs Shivkumar and Ardell), and the National Heart, Lung, and Blood Institute Grants F32 HL160163 (Dr Hadaya) and R01 HL159001 (Drs Ajijola, Shivkumar, and Ardell). University of California-Los Angeles has patents developed by Drs Ardell and Shivkumar relating to cardiac neural diagnostics and therapeutics. Drs Ardell, Ajijola, and Shivkumar are cofounders of NeuCures, Inc. All other authors have no relationships relevant to the contents of this paper to disclose.
Figures

Similar articles
-
Semaphorin 3a transfection into the left stellate ganglion reduces susceptibility to ventricular arrhythmias after myocardial infarction in rats.Europace. 2016 Dec;18(12):1886-1896. doi: 10.1093/europace/euv276. Epub 2015 Nov 4. Europace. 2016. PMID: 26541708
-
Reduced Cell Excitability of Cardiac Postganglionic Parasympathetic Neurons Correlates With Myocardial Infarction-Induced Fatal Ventricular Arrhythmias in Type 2 Diabetes Mellitus.Front Neurosci. 2021 Aug 18;15:721364. doi: 10.3389/fnins.2021.721364. eCollection 2021. Front Neurosci. 2021. PMID: 34483832 Free PMC article.
-
Effects of neuregulin-1 on autonomic nervous system remodeling post-myocardial infarction in a rat model.Neural Regen Res. 2017 Nov;12(11):1905-1910. doi: 10.4103/1673-5374.219054. Neural Regen Res. 2017. PMID: 29239338 Free PMC article.
-
Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death.Cardiovasc Res. 2001 May;50(2):409-16. doi: 10.1016/s0008-6363(00)00308-4. Cardiovasc Res. 2001. PMID: 11334845 Review.
-
Cardiac Sympathetic Nerve Sprouting and Susceptibility to Ventricular Arrhythmias after Myocardial Infarction.Cardiol Res Pract. 2015;2015:698368. doi: 10.1155/2015/698368. Epub 2015 Dec 17. Cardiol Res Pract. 2015. PMID: 26793403 Free PMC article. Review.
Cited by
-
Vagus Nerve Stimulation for Myocardial Ischemia: The Sooner the Better.JACC Basic Transl Sci. 2023 Sep 25;8(9):1119-1122. doi: 10.1016/j.jacbts.2023.07.007. eCollection 2023 Sep. JACC Basic Transl Sci. 2023. PMID: 37791308 Free PMC article.
-
Outcomes of hypothalamic oxytocin neuron-driven cardioprotection after acute myocardial infarction.Basic Res Cardiol. 2023 Oct 6;118(1):43. doi: 10.1007/s00395-023-01013-1. Basic Res Cardiol. 2023. PMID: 37801130 Free PMC article.
-
Advances in preclinical surgical therapy of cardiovascular diseases.Int J Surg. 2024 Aug 1;110(8):4965-4975. doi: 10.1097/JS9.0000000000001534. Int J Surg. 2024. PMID: 38701509 Free PMC article. Review.
-
Comparative specialization of intrinsic cardiac neurons in humans, mice, and pigs.bioRxiv [Preprint]. 2024 Apr 8:2024.04.04.588174. doi: 10.1101/2024.04.04.588174. bioRxiv. 2024. Update in: J Physiol. 2024 Nov 8. doi: 10.1113/JP286714. PMID: 38645175 Free PMC article. Updated. Preprint.
-
Vagal nerve stimulation in myocardial ischemia/reperfusion injury: from bench to bedside.Bioelectron Med. 2024 Sep 13;10(1):22. doi: 10.1186/s42234-024-00153-6. Bioelectron Med. 2024. PMID: 39267134 Free PMC article. Review.
References
-
- Benjamin E.J., Virani S.S., Callaway C.W., et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. - PubMed
-
- Ibanez B., James S., Agewall S., et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–177. - PubMed

