Viral dissemination and immune activation modulate antiretroviral drug levels in lymph nodes of SIV-infected rhesus macaques
- PMID: 37790938
- PMCID: PMC10544331
- DOI: 10.3389/fimmu.2023.1213455
Viral dissemination and immune activation modulate antiretroviral drug levels in lymph nodes of SIV-infected rhesus macaques
Abstract
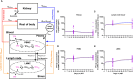
Introduction and methods: To understand the relationship between immunovirological factors and antiretroviral (ARV) drug levels in lymph nodes (LN) in HIV therapy, we analyzed drug levels in twenty-one SIV-infected rhesus macaques subcutaneously treated with daily tenofovir (TFV) and emtricitabine (FTC) for three months.
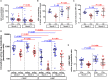
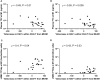
Results: The intracellular active drug-metabolite (IADM) levels (TFV-dp and FTC-tp) in lymph node mononuclear cells (LNMC) were significantly lower than in peripheral blood mononuclear cells (PBMC) (P≤0.005). Between Month 1 and Month 3, IADM levels increased in both LNMC (P≤0.001) and PBMC (P≤0.01), with a steeper increase in LNMC (P≤0.01). The viral dissemination in plasma, LN, and rectal tissue at ART initiation correlated negatively with IADM levels at Month 1. Physiologically-based pharmacokinetic model simulations suggest that, following subcutaneous ARV administration, ART-induced reduction of immune activation improves the formation of active drug-metabolites through modulation of kinase activity and/or through improved parent drug accessibility to LN cellular compartments.
Conclusion: These observations have broad implications for drugs that need to phosphorylate to exert their pharmacological activity, especially in the settings of the pre-/post-exposure prophylaxis and efficacy of antiviral therapies targeting pathogenic viruses such as HIV or SARS-CoV-2 replicating in highly inflammatory anatomic compartments.
Keywords: SIV infection; antiretroviral therapy (ART); drug metabolite; immune activation; lymph nodes; pharmacokinetic model; rhesus macaque; tenofovir.
Copyright © 2023 Srinivasula, Degrange, Perazzolo, Bonvillain, Tobery, Kaplan, Jang, Turnier, Davies, Cottrell, Ho and Di Mascio.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Training rhesus macaques to take daily oral antiretroviral therapy for preclinical evaluation of HIV prevention and treatment strategies.PLoS One. 2019 Nov 15;14(11):e0225146. doi: 10.1371/journal.pone.0225146. eCollection 2019. PLoS One. 2019. PMID: 31730629 Free PMC article.
-
Impact of Early ARV Initiation on Relative Proportions of Effector and Regulatory CD8 T Cell in Mesenteric Lymph Nodes and Peripheral Blood During Acute SIV Infection of Rhesus Macaques.J Virol. 2022 Apr 13;96(7):e0025522. doi: 10.1128/jvi.00255-22. Epub 2022 Mar 21. J Virol. 2022. PMID: 35311550 Free PMC article.
-
Persistent Viral Reservoirs in Lymphoid Tissues in SIV-Infected Rhesus Macaques of Chinese-Origin on Suppressive Antiretroviral Therapy.Viruses. 2019 Jan 27;11(2):105. doi: 10.3390/v11020105. Viruses. 2019. PMID: 30691203 Free PMC article.
-
Tenofovir diphosphate and emtricitabine triphosphate concentrations in blood cells compared with isolated peripheral blood mononuclear cells: a new measure of antiretroviral adherence?J Acquir Immune Defic Syndr. 2013 Mar 1;62(3):260-6. doi: 10.1097/QAI.0b013e3182794723. J Acquir Immune Defic Syndr. 2013. PMID: 23111578 Free PMC article.
-
Antiretroviral agents in pre-exposure prophylaxis: emerging and advanced trends in HIV prevention.J Pharm Pharmacol. 2019 Sep;71(9):1339-1352. doi: 10.1111/jphp.13107. Epub 2019 May 29. J Pharm Pharmacol. 2019. PMID: 31144296 Review.
Cited by
-
Physiologically based Pharmacokinetic Model Validated to Enable Predictions Of Multiple Drugs in a Long-acting Drug-combination Nano-Particles (DcNP): Confirmation with 3 HIV Drugs, Lopinavir, Ritonavir, and Tenofovir in DcNP Products.J Pharm Sci. 2024 Jun;113(6):1653-1663. doi: 10.1016/j.xphs.2024.02.018. Epub 2024 Feb 20. J Pharm Sci. 2024. PMID: 38382809
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

