Sgo1 interacts with CENP-A to guide accurate chromosome segregation in mitosis
- PMID: 37777834
- PMCID: PMC11181942
- DOI: 10.1093/jmcb/mjad061
Sgo1 interacts with CENP-A to guide accurate chromosome segregation in mitosis
Abstract
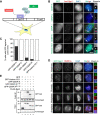
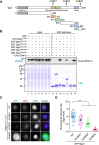
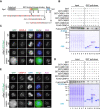
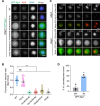
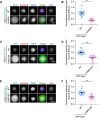
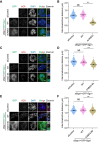
Shugoshin-1 (Sgo1) is necessary for maintaining sister centromere cohesion and ensuring accurate chromosome segregation during mitosis. It has been reported that the localization of Sgo1 at the centromere is dependent on Bub1-mediated phosphorylation of histone H2A at T120. However, it remains uncertain whether other centromeric proteins play a role in regulating the localization and function of Sgo1 during mitosis. Here, we show that CENP-A interacts with Sgo1 and determines the localization of Sgo1 to the centromere during mitosis. Further biochemical characterization revealed that lysine and arginine residues in the C-terminal domain of Sgo1 are critical for binding CENP-A. Interestingly, the replacement of these basic amino acids with acidic amino acids perturbed the localization of Sgo1 and Aurora B to the centromere, resulting in aberrant chromosome segregation and premature chromatid separation. Taken together, these findings reveal a previously unrecognized but direct link between Sgo1 and CENP-A in centromere plasticity control and illustrate how the Sgo1-CENP-A interaction guides accurate cell division.
Keywords: Aurora B; CENP-A; Sgo1; centromere; kinetochore; mitosis.
© The Author(s) (2023). Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, CEMCS, CAS.
Figures
Similar articles
-
Aurora B promotes the CENP-T-CENP-W interaction to guide accurate chromosome segregation in mitosis.J Mol Cell Biol. 2024 Jul 29;16(2):mjae001. doi: 10.1093/jmcb/mjae001. J Mol Cell Biol. 2024. PMID: 38200711 Free PMC article.
-
Posttranslational modifications of CENP-A: marks of distinction.Chromosoma. 2018 Sep;127(3):279-290. doi: 10.1007/s00412-018-0665-x. Epub 2018 Mar 22. Chromosoma. 2018. PMID: 29569072 Free PMC article. Review.
-
Transcription of a centromere-enriched retroelement and local retention of its RNA are significant features of the CENP-A chromatin landscape.Genome Biol. 2024 Nov 18;25(1):295. doi: 10.1186/s13059-024-03433-1. Genome Biol. 2024. PMID: 39558354 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Biphasic incorporation of centromeric histone CENP-A in fission yeast.Mol Biol Cell. 2008 Feb;19(2):682-90. doi: 10.1091/mbc.e07-05-0504. Epub 2007 Dec 12. Mol Biol Cell. 2008. PMID: 18077559 Free PMC article.
Cited by
-
Novel insights into the circadian modulation of lipid metabolism in chicken livers revealed by RNA sequencing and weighted gene co-expression network analysis.Poult Sci. 2024 Dec;103(12):104321. doi: 10.1016/j.psj.2024.104321. Epub 2024 Sep 16. Poult Sci. 2024. PMID: 39361997 Free PMC article.
References
-
- Cheeseman I.M., Desai A. (2008). Molecular architecture of the kinetochore–microtubule interface. Nat. Rev. Mol. Cell Biol. 9, 33–46. - PubMed

