Decreased myelin-related gene expression in the nucleus accumbens during spontaneous neonatal opioid withdrawal in the absence of long-term behavioral effects in adult outbred CFW mice
- PMID: 37774943
- PMCID: PMC10598517
- DOI: 10.1016/j.neuropharm.2023.109732
Decreased myelin-related gene expression in the nucleus accumbens during spontaneous neonatal opioid withdrawal in the absence of long-term behavioral effects in adult outbred CFW mice
Abstract
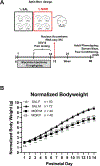
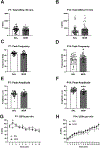
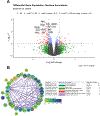
Prenatal opioid exposure is a major health concern in the United States, with the incidence of neonatal opioid withdrawal syndrome (NOWS) escalating in recent years. NOWS occurs upon cessation of in utero opioid exposure and is characterized by increased irritability, disrupted sleep patterns, high-pitched crying, and dysregulated feeding. The main pharmacological strategy for alleviating symptoms is treatment with replacement opioids. The neural mechanisms mediating NOWS and the long-term neurobehavioral effects are poorly understood. We used a third trimester-approximate model in which neonatal outbred pups (Carworth Farms White; CFW) were administered once-daily morphine (15 mg/kg, s.c.) from postnatal day (P) day 1 through P14 and were then assessed for behavioral and transcriptomic adaptations within the nucleus accumbens (NAc) on P15. We also investigated the long-term effects of perinatal morphine exposure on adult learning and reward sensitivity. We observed significant weight deficits, spontaneous thermal hyperalgesia, and altered ultrasonic vocalization (USV) profiles following repeated morphine and during spontaneous withdrawal. Transcriptome analysis of NAc from opioid-withdrawn P15 neonates via bulk mRNA sequencing identified an enrichment profile consistent with downregulation of myelin-associated transcripts. Despite the neonatal behavioral and molecular effects, there were no significant long-term effects of perinatal morphine exposure on adult spatial memory function in the Barnes Maze, emotional learning in fear conditioning, or in baseline or methamphetamine-potentiated reward sensitivity as measured via intracranial self-stimulation. Thus, the once daily third trimester-approximate exposure regimen, while inducing NOWS model traits and significant transcriptomic effects in neonates, had no significant long-term effects on adult behaviors.
Keywords: ICSS; Neonatal abstinence syndrome; Neonatal opioid withdrawal syndrome; Opiate; Opioid use disorder; RNA-seq; Swiss webster.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest This work was supported by U01DA050243. We have no conflicts of interest.
Figures
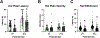
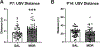

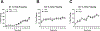

Update of
-
Decreased myelin-related gene expression in the nucleus accumbens during spontaneous neonatal opioid withdrawal in the absence of long-term behavioral effects in adult outbred CFW mice.bioRxiv [Preprint]. 2023 Aug 7:2023.08.04.552033. doi: 10.1101/2023.08.04.552033. bioRxiv. 2023. Update in: Neuropharmacology. 2023 Dec 1;240:109732. doi: 10.1016/j.neuropharm.2023.109732. PMID: 37609129 Free PMC article. Updated. Preprint.
Similar articles
-
The acoustic properties, syllable structure, and syllable sequences of ultrasonic vocalizations (USVs) during neonatal opioid withdrawal in FVB/N mouse substrains.bioRxiv [Preprint]. 2024 Nov 7:2024.11.06.622304. doi: 10.1101/2024.11.06.622304. bioRxiv. 2024. PMID: 39574631 Free PMC article. Preprint.
-
Decreased myelin-related gene expression in the nucleus accumbens during spontaneous neonatal opioid withdrawal in the absence of long-term behavioral effects in adult outbred CFW mice.bioRxiv [Preprint]. 2023 Aug 7:2023.08.04.552033. doi: 10.1101/2023.08.04.552033. bioRxiv. 2023. Update in: Neuropharmacology. 2023 Dec 1;240:109732. doi: 10.1016/j.neuropharm.2023.109732. PMID: 37609129 Free PMC article. Updated. Preprint.
-
Maternal opioid use during pregnancy and the risk of neonatal opioid withdrawal syndrome in the offspring.Acta Obstet Gynecol Scand. 2024 Aug;103(8):1522-1529. doi: 10.1111/aogs.14850. Epub 2024 May 3. Acta Obstet Gynecol Scand. 2024. PMID: 38700023 Free PMC article.
-
Neonatal opioid toxicity: opioid withdrawal (abstinence) syndrome with emphasis on pharmacogenomics and respiratory depression.Arch Toxicol. 2023 Oct;97(10):2575-2585. doi: 10.1007/s00204-023-03563-8. Epub 2023 Aug 3. Arch Toxicol. 2023. PMID: 37537419 Review.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
Cited by
-
Advances in animal models of prenatal opioid exposure.Trends Neurosci. 2024 May;47(5):367-382. doi: 10.1016/j.tins.2024.03.005. Epub 2024 Apr 12. Trends Neurosci. 2024. PMID: 38614891 Review.
References
-
- Bakhireva Ludmila N., Holbrook Bradley D., Shrestha Shikhar, Leyva Yuridia, Ashley Malia, Cano Sandra, Lowe Jean, Stephen Julia M., and Leeman Lawrence. 2019. “Association between Prenatal Opioid Exposure, Neonatal Opioid Withdrawal Syndrome, and Neurodevelopmental and Behavioral Outcomes at 5–8-Months of Age.” Early Human Development 128 (January): 69–76. 10.1016/j.earlhumdev.2018.10.010. - DOI - PMC - PubMed
-
- Bauer Charles R., Langer John, Lambert-Brown Brittany, Shankaran Seetha, Bada Henrietta S., Lester Barry, Lagasse Lynn L., Whitaker Toni, and Hammond Jane. 2020. “Association of Prenatal Opiate Exposure with Youth Outcomes Assessed from Infancy through Adolescence.” Journal of Perinatology: Official Journal of the California Perinatal Association 40 (7): 1056–65. 10.1038/s41372-020-0692-3. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

