The intricate role of CCL5/CCR5 axis in Alzheimer disease
- PMID: 37769321
- PMCID: PMC10587995
- DOI: 10.1093/jnen/nlad071
The intricate role of CCL5/CCR5 axis in Alzheimer disease
Abstract
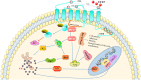
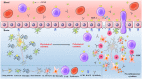
The morbidity and mortality associated with Alzheimer disease (AD), one of the most common neurodegenerative diseases, are increasing each year. Although both amyloid β and tau proteins are known to be involved in AD pathology, their detailed functions in the pathogenesis of the disease are not fully understood. There is increasing evidence that neuroinflammation contributes to the development and progression of AD, with astrocytes, microglia, and the cytokines and chemokines they secrete acting coordinately in these processes. Signaling involving chemokine (C-C motif) ligand 5 (CCL5) and its main receptor C-C chemokine receptor 5 (CCR5) plays an important role in normal physiologic processes as well as pathologic conditions such as neurodegeneration. In recent years, many studies have shown that the CCL5/CCR5 axis plays a major effect in the pathogenesis of AD, but there are also a few studies that contradict this. In short, the role of CCL5/CCR5 axis in the pathogenesis of AD is still intricate. This review summarizes the structure, distribution, physiologic functions of the CCL5/CCR5 axis, and the progress in understanding its involvement in the pathogenesis of AD.
Keywords: Alzheimer disease; Axis; CCL5; CCR5; Neuroinflammation.
© The Author(s) 2023. Published by Oxford University Press on behalf of American Association of Neuropathologists, Inc.
Conflict of interest statement
The authors have no duality or conflicts of interest to declare.
Figures
Similar articles
-
Expression and biological functions of the CCL5-CCR5 axis in oral lichen planus.Exp Dermatol. 2019 Jul;28(7):816-821. doi: 10.1111/exd.13946. Epub 2019 May 15. Exp Dermatol. 2019. PMID: 31006151
-
Inhibition of the CCL5/CCR5 Axis against the Progression of Gastric Cancer.Int J Mol Sci. 2018 May 16;19(5):1477. doi: 10.3390/ijms19051477. Int J Mol Sci. 2018. PMID: 29772686 Free PMC article. Review.
-
Immunohistochemical study of the beta-chemokine receptors CCR3 and CCR5 and their ligands in normal and Alzheimer's disease brains.Am J Pathol. 1998 Jul;153(1):31-7. doi: 10.1016/s0002-9440(10)65542-3. Am J Pathol. 1998. PMID: 9665462 Free PMC article.
-
Peripheral chemokine receptors, their ligands, cytokines and Alzheimer's disease.J Alzheimers Dis. 2008 Jun;14(2):147-59. doi: 10.3233/jad-2008-14203. J Alzheimers Dis. 2008. PMID: 18560127
-
The potential to target CCL5/CCR5 in breast cancer.Expert Opin Ther Targets. 2014 Nov;18(11):1265-75. doi: 10.1517/14728222.2014.949238. Epub 2014 Sep 26. Expert Opin Ther Targets. 2014. PMID: 25256399 Review.
Cited by
-
mosGraphFlow: a novel integrative graph AI model mining disease targets from multi-omic data.bioRxiv [Preprint]. 2024 Sep 3:2024.08.01.606219. doi: 10.1101/2024.08.01.606219. bioRxiv. 2024. PMID: 39282361 Free PMC article. Preprint.
-
Transcriptome analysis of the aged SAMP8 mouse model of Alzheimer's disease reveals novel molecular targets of formononetin protection.Front Pharmacol. 2024 Aug 21;15:1440515. doi: 10.3389/fphar.2024.1440515. eCollection 2024. Front Pharmacol. 2024. PMID: 39234102 Free PMC article.
-
Spotlight on pro-inflammatory chemokines: regulators of cellular communication in cognitive impairment.Front Immunol. 2024 Jul 1;15:1421076. doi: 10.3389/fimmu.2024.1421076. eCollection 2024. Front Immunol. 2024. PMID: 39011039 Free PMC article. Review.
References
-
- Jia L, Du Y, Chu L, et al.; COAST Group. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: A cross-sectional study. Lancet Public Health 2020;5:e661–71 - PubMed
-
- Braak H, Braak E.. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–59 - PubMed
-
- Webers A, Heneka MT, Gleeson PA.. The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol Cell Biol 2020;98:28–41 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

