Transcriptional Stochasticity as a Key Aspect of HIV-1 Latency
- PMID: 37766375
- PMCID: PMC10535884
- DOI: 10.3390/v15091969
Transcriptional Stochasticity as a Key Aspect of HIV-1 Latency
Abstract
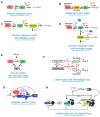
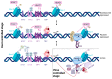
This review summarizes current advances in the role of transcriptional stochasticity in HIV-1 latency, which were possible in a large part due to the development of single-cell approaches. HIV-1 transcription proceeds in bursts of RNA production, which stem from the stochastic switching of the viral promoter between ON and OFF states. This switching is caused by random binding dynamics of transcription factors and nucleosomes to the viral promoter and occurs at several time scales from minutes to hours. Transcriptional bursts are mainly controlled by the core transcription factors TBP, SP1 and NF-κb, the chromatin status of the viral promoter and RNA polymerase II pausing. In particular, spontaneous variability in the promoter chromatin creates heterogeneity in the response to activators such as TNF-α, which is then amplified by the Tat feedback loop to generate high and low viral transcriptional states. This phenomenon is likely at the basis of the partial and stochastic response of latent T cells from HIV-1 patients to latency-reversing agents, which is a barrier for the development of shock-and-kill strategies of viral eradication. A detailed understanding of the transcriptional stochasticity of HIV-1 and the possibility to precisely model this phenomenon will be important assets to develop more effective therapeutic strategies.
Keywords: HIV-1 latency; chromatin; integration site; modeling; promoter; transcription; transcription factor; transcriptional burst; transcriptional noise.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Control of stochastic gene expression by host factors at the HIV promoter.PLoS Pathog. 2009 Jan;5(1):e1000260. doi: 10.1371/journal.ppat.1000260. Epub 2009 Jan 9. PLoS Pathog. 2009. PMID: 19132086 Free PMC article.
-
Semen Exosomes Promote Transcriptional Silencing of HIV-1 by Disrupting NF-κB/Sp1/Tat Circuitry.J Virol. 2018 Oct 12;92(21):e00731-18. doi: 10.1128/JVI.00731-18. Print 2018 Nov 1. J Virol. 2018. PMID: 30111566 Free PMC article.
-
Enhanced Transcriptional Strength of HIV-1 Subtype C Minimizes Gene Expression Noise and Confers Stability to the Viral Latent State.J Virol. 2023 Jan 31;97(1):e0137622. doi: 10.1128/jvi.01376-22. Epub 2022 Dec 19. J Virol. 2023. PMID: 36533949 Free PMC article.
-
Chromatin-associated regulation of HIV-1 transcription: implications for the development of therapeutic strategies.Subcell Biochem. 2007;41:371-96. Subcell Biochem. 2007. PMID: 17484137 Review.
-
Transcription: Insights From the HIV-1 Promoter.Int Rev Cell Mol Biol. 2018;335:191-243. doi: 10.1016/bs.ircmb.2017.07.011. Epub 2017 Oct 12. Int Rev Cell Mol Biol. 2018. PMID: 29305013 Review.
Cited by
-
Stochastic Gene Expression in Proliferating Cells: Differing Noise Intensity in Single-Cell and Population Perspectives.bioRxiv [Preprint]. 2024 Jun 29:2024.06.28.601263. doi: 10.1101/2024.06.28.601263. bioRxiv. 2024. PMID: 38979195 Free PMC article. Preprint.
-
Identification of molecular determinants of gene-specific bursting patterns by high-throughput imaging screens.bioRxiv [Preprint]. 2024 Jun 8:2024.06.08.597999. doi: 10.1101/2024.06.08.597999. bioRxiv. 2024. PMID: 38903099 Free PMC article. Preprint.
-
The cell biology of HIV-1 latency and rebound.Retrovirology. 2024 Apr 5;21(1):6. doi: 10.1186/s12977-024-00639-w. Retrovirology. 2024. PMID: 38580979 Free PMC article. Review.
-
Mathematical Models of HIV-1 Dynamics, Transcription, and Latency.Viruses. 2023 Oct 19;15(10):2119. doi: 10.3390/v15102119. Viruses. 2023. PMID: 37896896 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

