Attachment, Entry, and Intracellular Trafficking of Classical Swine Fever Virus
- PMID: 37766277
- PMCID: PMC10534341
- DOI: 10.3390/v15091870
Attachment, Entry, and Intracellular Trafficking of Classical Swine Fever Virus
Abstract
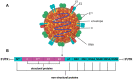
Classical swine fever virus (CSFV), which is a positive-sense, single-stranded RNA virus with an envelope, is a member of the Pestivirus genus in the Flaviviridae family. CSFV causes a severe and highly contagious disease in pigs and is prevalent worldwide, threatening the pig farming industry. The detailed mechanisms of the CSFV life cycle have been reported, but are still limited. Some receptors and attachment factors of CSFV, including heparan sulfate (HS), laminin receptor (LamR), complement regulatory protein (CD46), MER tyrosine kinase (MERTK), disintegrin, and metalloproteinase domain-containing protein 17 (ADAM17), were identified. After attachment, CSFV internalizes via clathrin-mediated endocytosis (CME) and/or caveolae/raft-dependent endocytosis (CavME). After internalization, CSFV moves to early and late endosomes before uncoating. During this period, intracellular trafficking of CSFV relies on components of the endosomal sorting complex required for transport (ESCRT) and Rab proteins in the endosome dynamics, with a dependence on the cytoskeleton network. This review summarizes the data on the mechanisms of CSFV attachment, internalization pathways, and intracellular trafficking, and provides a general view of the early events in the CSFV life cycle.
Keywords: attachment; classical swine fever virus; entry; intracellular trafficking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The ESCRT-I Subunit Tsg101 Plays Novel Dual Roles in Entry and Replication of Classical Swine Fever Virus.J Virol. 2021 Feb 24;95(6):e01928-20. doi: 10.1128/JVI.01928-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33328308 Free PMC article.
-
Rab5, Rab7, and Rab11 Are Required for Caveola-Dependent Endocytosis of Classical Swine Fever Virus in Porcine Alveolar Macrophages.J Virol. 2018 Jul 17;92(15):e00797-18. doi: 10.1128/JVI.00797-18. Print 2018 Aug 1. J Virol. 2018. PMID: 29769350 Free PMC article.
-
Entry of Classical Swine Fever Virus into PK-15 Cells via a pH-, Dynamin-, and Cholesterol-Dependent, Clathrin-Mediated Endocytic Pathway That Requires Rab5 and Rab7.J Virol. 2016 Sep 29;90(20):9194-208. doi: 10.1128/JVI.00688-16. Print 2016 Oct 15. J Virol. 2016. PMID: 27489278 Free PMC article.
-
Host cell factors involved in classical swine fever virus entry.Vet Res. 2023 Dec 1;54(1):115. doi: 10.1186/s13567-023-01238-x. Vet Res. 2023. PMID: 38041163 Free PMC article. Review.
-
Genetic variability and distribution of Classical swine fever virus.Anim Health Res Rev. 2015 Jun;16(1):33-9. doi: 10.1017/S1466252315000109. Anim Health Res Rev. 2015. PMID: 26050570 Review.
Cited by
-
Comprehensive Characterization of the Genetic Landscape of African Swine Fever Virus: Insights into Infection Dynamics, Immunomodulation, Virulence and Genes with Unknown Function.Animals (Basel). 2024 Jul 26;14(15):2187. doi: 10.3390/ani14152187. Animals (Basel). 2024. PMID: 39123713 Free PMC article. Review.
-
Porcine low-density lipoprotein receptor plays an important role in classical swine fever virus infection.Emerg Microbes Infect. 2024 Dec;13(1):2327385. doi: 10.1080/22221751.2024.2327385. Epub 2024 Mar 21. Emerg Microbes Infect. 2024. PMID: 38514916 Free PMC article.
-
Significance of Artificial Intelligence in the Study of Virus-Host Cell Interactions.Biomolecules. 2024 Jul 26;14(8):911. doi: 10.3390/biom14080911. Biomolecules. 2024. PMID: 39199298 Free PMC article. Review.
-
Classical swine fever: Unveiling the complexity through a multifaceted approach.Open Vet J. 2024 Oct;14(10):2497-2508. doi: 10.5455/OVJ.2024.v14.i10.1. Epub 2024 Oct 31. Open Vet J. 2024. PMID: 39545196 Free PMC article. Review.
References
-
- Meyers G., Thiel H.J. Molecular characterization of pestiviruses. Adv. Virus Res. 1996;47:53–118. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

