Feline Infectious Peritonitis: European Advisory Board on Cat Diseases Guidelines
- PMID: 37766254
- PMCID: PMC10535984
- DOI: 10.3390/v15091847
Feline Infectious Peritonitis: European Advisory Board on Cat Diseases Guidelines
Abstract
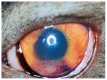
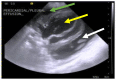
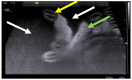
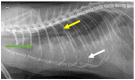
Feline coronavirus (FCoV) is a ubiquitous RNA virus of cats, which is transmitted faeco-orally. In these guidelines, the European Advisory Board on Cat Diseases (ABCD) presents a comprehensive review of feline infectious peritonitis (FIP). FCoV is primarily an enteric virus and most infections do not cause clinical signs, or result in only enteritis, but a small proportion of FCoV-infected cats develop FIP. The pathology in FIP comprises a perivascular phlebitis that can affect any organ. Cats under two years old are most frequently affected by FIP. Most cats present with fever, anorexia, and weight loss; many have effusions, and some have ocular and/or neurological signs. Making a diagnosis is complex and ABCD FIP Diagnostic Approach Tools are available to aid veterinarians. Sampling an effusion, when present, for cytology, biochemistry, and FCoV RNA or FCoV antigen detection is very useful diagnostically. In the absence of an effusion, fine-needle aspirates from affected organs for cytology and FCoV RNA or FCoV antigen detection are helpful. Definitive diagnosis usually requires histopathology with FCoV antigen detection. Antiviral treatments now enable recovery in many cases from this previously fatal disease; nucleoside analogues (e.g., oral GS-441524) are very effective, although they are not available in all countries.
Keywords: FCoV; FIP; antiviral; diagnosis; feline coronavirus; mutation; treatment.
Conflict of interest statement
The authors are members of the European Advisory Board on Cat Diseases (ABCD), a scientifically independent board of experts in feline medicine. Some individual authors have been consultants for, or have received research funding and honoraria from, animal health companies and other organisations, but all authors declare no conflicts of interest for the writing of these independent guidelines. The ABCD sponsors had no role in the writing, nor in the decision to publish this review.
Figures











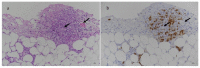


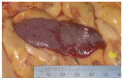



Similar articles
-
Sensitivity and specificity of a real-time reverse transcriptase polymerase chain reaction detecting feline coronavirus mutations in effusion and serum/plasma of cats to diagnose feline infectious peritonitis.BMC Vet Res. 2017 Aug 2;13(1):228. doi: 10.1186/s12917-017-1147-8. BMC Vet Res. 2017. PMID: 28768514 Free PMC article.
-
Diagnosis of non-effusive feline infectious peritonitis by reverse transcriptase quantitative PCR from mesenteric lymph node fine-needle aspirates.J Feline Med Surg. 2019 Oct;21(10):910-921. doi: 10.1177/1098612X18809165. Epub 2018 Nov 8. J Feline Med Surg. 2019. PMID: 30407137 Free PMC article.
-
Long-term follow-up of cats in complete remission after treatment of feline infectious peritonitis with oral GS-441524.J Feline Med Surg. 2023 Aug;25(8):1098612X231183250. doi: 10.1177/1098612X231183250. J Feline Med Surg. 2023. PMID: 37548535 Free PMC article.
-
Feline infectious peritonitis. ABCD guidelines on prevention and management.J Feline Med Surg. 2009 Jul;11(7):594-604. doi: 10.1016/j.jfms.2009.05.008. J Feline Med Surg. 2009. PMID: 19481039 Free PMC article. Review.
-
2022 AAFP/EveryCat Feline Infectious Peritonitis Diagnosis Guidelines.J Feline Med Surg. 2022 Sep;24(9):905-933. doi: 10.1177/1098612X221118761. J Feline Med Surg. 2022. PMID: 36002137 Free PMC article. Review.
Cited by
-
Causes of shelter cats mortality in the Czech Republic.Vet Anim Sci. 2024 Jul 17;25:100379. doi: 10.1016/j.vas.2024.100379. eCollection 2024 Sep. Vet Anim Sci. 2024. PMID: 39157703 Free PMC article.
-
Detection of Feline Coronavirus in Bronchoalveolar Lavage Fluid from Cats with Atypical Lower Airway and Lung Disease: Suspicion of Virus-Associated Pneumonia or Pneumonitis.Animals (Basel). 2024 Apr 18;14(8):1219. doi: 10.3390/ani14081219. Animals (Basel). 2024. PMID: 38672364 Free PMC article.
-
Short Treatment of 42 Days with Oral GS-441524 Results in Equal Efficacy as the Recommended 84-Day Treatment in Cats Suffering from Feline Infectious Peritonitis with Effusion-A Prospective Randomized Controlled Study.Viruses. 2024 Jul 16;16(7):1144. doi: 10.3390/v16071144. Viruses. 2024. PMID: 39066306 Free PMC article.
-
Detection of feline coronavirus RNA, spike gene mutations, and feline coronavirus antigen in macrophages in aqueous humor of cats in the diagnosis of feline infectious peritonitis.J Vet Diagn Invest. 2020 Jul;32(4):527-534. doi: 10.1177/1040638720927362. Epub 2020 Jun 9. J Vet Diagn Invest. 2020. PMID: 32517543 Free PMC article.
-
Assessing the feasibility of applying machine learning to diagnosing non-effusive feline infectious peritonitis.Sci Rep. 2024 Jan 30;14(1):2517. doi: 10.1038/s41598-024-52577-4. Sci Rep. 2024. PMID: 38291072 Free PMC article.
References
-
- Pedersen N.C., Boyle J.F., Floyd K., Fudge A., Barker J. An enteric coronavirus infection of cats and its relationship to feline infectious peritonitis. Am. J. Vet. Res. 1981;42:368–377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

