Structural and Synthetic Aspects of Small Ring Oxa- and Aza-Heterocyclic Ring Systems as Antiviral Activities
- PMID: 37766233
- PMCID: PMC10536032
- DOI: 10.3390/v15091826
Structural and Synthetic Aspects of Small Ring Oxa- and Aza-Heterocyclic Ring Systems as Antiviral Activities
Abstract
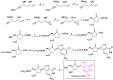
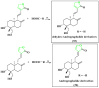
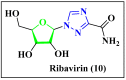
Antiviral properties of different oxa- and aza-heterocycles are identified and properly correlated with their structural features and discussed in this review article. The primary objective is to explore the activity of such ring systems as antiviral agents, as well as their synthetic routes and biological significance. Eventually, the structure-activity relationship (SAR) of the heterocyclic compounds, along with their salient characteristics are exhibited to build a suitable platform for medicinal chemists and biotechnologists. The synergistic conclusions are extremely important for the introduction of a newer tool for the future drug discovery program.
Keywords: Structure Activity Relationship (SAR); antiviral agents; heterocycles; natural products; synthetic methods.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


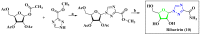

Similar articles
-
Antiviral Activities of Pyridine Fused and Pyridine Containing Heterocycles, A Review (from 2000 to 2020).Mini Rev Med Chem. 2021;21(17):2584-2611. doi: 10.2174/1389557521666210126143558. Mini Rev Med Chem. 2021. PMID: 33573543 Review.
-
Bioactive pyrrole-based compounds with target selectivity.Eur J Med Chem. 2020 Dec 15;208:112783. doi: 10.1016/j.ejmech.2020.112783. Epub 2020 Aug 29. Eur J Med Chem. 2020. PMID: 32916311 Free PMC article. Review.
-
Discovery and optimization of potent broad-spectrum arenavirus inhibitors derived from benzimidazole and related heterocycles.Bioorg Med Chem Lett. 2013 Feb 1;23(3):750-6. doi: 10.1016/j.bmcl.2012.11.093. Epub 2012 Dec 1. Bioorg Med Chem Lett. 2013. PMID: 23265900
-
A review on antioxidant potential of bioactive heterocycle benzofuran: Natural and synthetic derivatives.Pharmacol Rep. 2017 Apr;69(2):281-295. doi: 10.1016/j.pharep.2016.11.007. Epub 2016 Nov 18. Pharmacol Rep. 2017. PMID: 28171830 Review.
-
Recent studies of nitrogen containing heterocyclic compounds as novel antiviral agents: A review.Bioorg Chem. 2021 Sep;114:105076. doi: 10.1016/j.bioorg.2021.105076. Epub 2021 Jun 10. Bioorg Chem. 2021. PMID: 34157555 Review.
Cited by
-
Novel Antiviral Agents: Synthesis, Molecular Modelling Studies and Biological Investigation.Viruses. 2023 Oct 2;15(10):2042. doi: 10.3390/v15102042. Viruses. 2023. PMID: 37896819 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

