A Subunit Vaccine Candidate Composed of Mpox Virus A29L, M1R, A35R, and B6R Elicits Robust Immune Response in Mice
- PMID: 37766097
- PMCID: PMC10537547
- DOI: 10.3390/vaccines11091420
A Subunit Vaccine Candidate Composed of Mpox Virus A29L, M1R, A35R, and B6R Elicits Robust Immune Response in Mice
Abstract
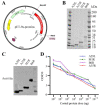
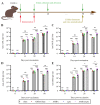
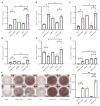
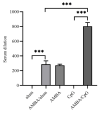
With no specific antiviral drugs and preventive vaccines against Mpox virus (MPXV), the epidemic has led to the declaration of a Public Health Emergency of International Concern. As a developmental direction for new vaccines, studies of subunit vaccines based upon MPXV antigen proteins are lacking. In this study, A29L, M1R, A35R, and B6R of MPXV were expressed and purified from a prokaryotic system. The four MPXV antigen proteins in combination were mixed with aluminum hydroxide or CpG7909 as adjuvant, and subsequently used to inoculate mice. The results of enzyme-linked immunosorbent assay (ELISA), flow cytometry analyses, and enzyme-linked immunospot (ELISPOT) assays indicated that A29L, M1R, A35R, and B6R elicited high-level antigen-specific antibodies and CD4+ T cells-based cellular immune response in mice. Moreover, the results of virus neutralization assays suggested that sera from the mice immunized with four proteins elicited high neutralizing activities against the vaccinia virus. Notably, the results of ELISA, ELISPOT, and virus neutralization assays also showed that the CpG7909 adjuvant was more effective in inducing an immune response compared with the aluminum adjuvant. In summary, this study offers valuable insights for further studies of subunit vaccine candidates for the prevention of MPXV and other orthomyxoviruses.
Keywords: CpG7909 adjuvant; Mpox virus; immune response; prokaryotic expression; subunit vaccine.
Conflict of interest statement
None of the authors have any actual or potential conflict of interest.
Figures
Similar articles
-
Evaluation and comparison of immune responses induced by two Mpox mRNA vaccine candidates in mice.J Med Virol. 2023 Oct;95(10):e29140. doi: 10.1002/jmv.29140. J Med Virol. 2023. PMID: 37800627
-
Recombinant proteins A29L, M1R, A35R, and B6R vaccination protects mice from mpox virus challenge.Front Immunol. 2023 Jun 26;14:1203410. doi: 10.3389/fimmu.2023.1203410. eCollection 2023. Front Immunol. 2023. PMID: 37435062 Free PMC article.
-
Rational development of multicomponent mRNA vaccine candidates against mpox.Emerg Microbes Infect. 2023 Dec;12(1):2192815. doi: 10.1080/22221751.2023.2192815. Emerg Microbes Infect. 2023. PMID: 36947428 Free PMC article.
-
Differences in pathogenicity among the mpox virus clades: impact on drug discovery and vaccine development.Trends Pharmacol Sci. 2023 Oct;44(10):719-739. doi: 10.1016/j.tips.2023.08.003. Epub 2023 Sep 4. Trends Pharmacol Sci. 2023. PMID: 37673695 Review.
-
Monkeypox (Mpox) requires continued surveillance, vaccines, therapeutics and mitigating strategies.Vaccine. 2023 May 11;41(20):3171-3177. doi: 10.1016/j.vaccine.2023.04.010. Epub 2023 Apr 21. Vaccine. 2023. PMID: 37088603 Free PMC article. Review.
Cited by
-
Longitudinal viral shedding and antibody response characteristics of men with acute infection of monkeypox virus: a prospective cohort study.Nat Commun. 2024 May 27;15(1):4488. doi: 10.1038/s41467-024-48754-8. Nat Commun. 2024. PMID: 38802350 Free PMC article.
-
A cross-sectional investigation of factors influencing mpox vaccine hesitancy for students in Southwest China.Hum Vaccin Immunother. 2024 Dec 31;20(1):2309704. doi: 10.1080/21645515.2024.2309704. Epub 2024 Feb 1. Hum Vaccin Immunother. 2024. PMID: 38300140 Free PMC article.
References
-
- World Health Organization. [(accessed on 4 July 2023)]; Available online: https://worldhealthorg.shinyapps.io/mpx_global/
Grants and funding
- 2020T130044ZX/The Postdoctoral Science Foundation of China
- KQTD20200909113758004/The Shenzhen Science and Technology Innovation Commission
- ZDSYS20200811142804014/The Shenzhen Key Laboratory of Prevention and the Treatment of Severe Infections
- SZXK045/The Shenzhen Key Medical Discipline Construction Fund
LinkOut - more resources
Full Text Sources
Research Materials

