Engaging Isatins and Amino Acids in Multicomponent One-Pot 1,3-Dipolar Cycloaddition Reactions-Easy Access to Structural Diversity
- PMID: 37764264
- PMCID: PMC10536439
- DOI: 10.3390/molecules28186488
Engaging Isatins and Amino Acids in Multicomponent One-Pot 1,3-Dipolar Cycloaddition Reactions-Easy Access to Structural Diversity
Abstract
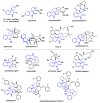
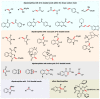
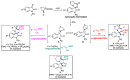
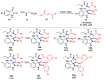
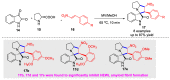
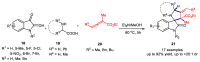
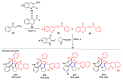
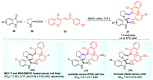
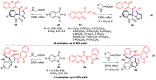
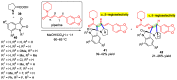
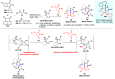
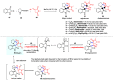
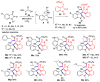
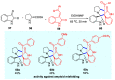
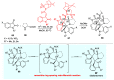
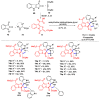
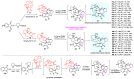
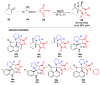
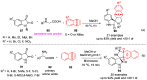
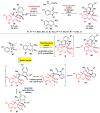
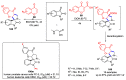
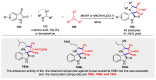
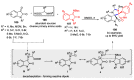
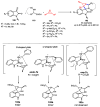
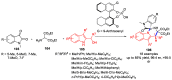
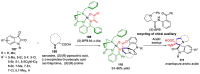
Multicomponent reactions (MCRs) have undoubtedly emerged as the most indispensable tool for organic chemists worldwide, finding extensive utility in the synthesis of intricate natural products, heterocyclic molecules with significant bioactivity, and pharmaceutical agents. The multicomponent one-pot 1,3-dipolar cycloaddition reactions, which were initially conceptualized by Rolf Huisgen in 1960, find extensive application in contemporary heterocyclic chemistry. In terms of green synthesis, the multicomponent 1,3-dipolar cycloaddition is highly favored owing to its numerous advantages, including high step- and atom-economies, remarkable product diversity, as well as excellent efficiency and diastereoselectivity. Among the numerous pieces of research, the most fascinating reaction involves the utilization of azomethine ylides generated from isatins and amino acids that can be captured by various dipolarophiles. This approach offers a highly efficient and convenient method for constructing spiro-pyrrolidine oxindole scaffolds, which are crucial building blocks in biologically active molecules. Consequently, this review delves deeper into the dipolarophiles utilized in the 1,3-dipolar cycloaddition of isatins and amino acids over the past six years.
Keywords: 1,3-dipolar cycloadditions; isatin; multicomponent reactions; one-pot reactions; spiro-oxindole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Catalytic enantioselective 1,3-dipolar cycloadditions of azomethine ylides for biology-oriented synthesis.Acc Chem Res. 2014 Apr 15;47(4):1296-310. doi: 10.1021/ar400286b. Epub 2014 Mar 22. Acc Chem Res. 2014. PMID: 24730692 Free PMC article.
-
Multicomponent Dipolar Cycloaddition Strategy: Combinatorial Synthesis of Novel Spiro-Tethered Pyrazolo[3,4-b]quinoline Hybrid Heterocycles.ACS Comb Sci. 2016 May 9;18(5):262-70. doi: 10.1021/acscombsci.6b00003. Epub 2016 Apr 8. ACS Comb Sci. 2016. PMID: 27027478
-
1,3-Dipolar cycloaddition reactions of isatin-derived azomethine ylides for the synthesis of spirooxindole and indole-derived scaffolds: recent developments.Mol Divers. 2023 Oct;27(5):2365-2397. doi: 10.1007/s11030-022-10510-9. Epub 2022 Aug 4. Mol Divers. 2023. PMID: 35925529 Review.
-
Brønsted-acid-catalyzed asymmetric multicomponent reactions for the facile synthesis of highly enantioenriched structurally diverse nitrogenous heterocycles.Acc Chem Res. 2011 Nov 15;44(11):1156-71. doi: 10.1021/ar2000343. Epub 2011 Jul 29. Acc Chem Res. 2011. PMID: 21800828
-
Isocyanide-Based Multicomponent Reactions in Water: Advanced Green Tools for the Synthesis of Heterocyclic Compounds.Top Curr Chem (Cham). 2022 Sep 22;380(6):50. doi: 10.1007/s41061-022-00403-8. Top Curr Chem (Cham). 2022. PMID: 36136281 Review.
Cited by
-
Silica/APTPOSS anchored on MnFe2O4 as an efficient nanomagnetic composite for the preparation of spiro-pyrano [2, 3-c] chromene derivatives.BMC Chem. 2024 Aug 24;18(1):155. doi: 10.1186/s13065-024-01270-8. BMC Chem. 2024. PMID: 39182154 Free PMC article.
-
Diastereoselective Synthesis of Dispiro[Imidazothiazolotriazine-Pyrrolidin-Oxindoles] and Their Isomerization Pathways in Basic Medium.Int J Mol Sci. 2023 Nov 15;24(22):16359. doi: 10.3390/ijms242216359. Int J Mol Sci. 2023. PMID: 38003560 Free PMC article.
References
-
- Cioc R.C., Ruijter E., Orru R.V.A. Multicomponent reactions: Advanced tools for sustainable organic synthesis. Green Chem. 2014;16:2958–2975. doi: 10.1039/C4GC00013G. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

