Novel Artificial Intelligence-Based Approaches for Ab Initio Structure Determination and Atomic Model Building for Cryo-Electron Microscopy
- PMID: 37763837
- PMCID: PMC10534518
- DOI: 10.3390/mi14091674
Novel Artificial Intelligence-Based Approaches for Ab Initio Structure Determination and Atomic Model Building for Cryo-Electron Microscopy
Abstract
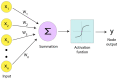
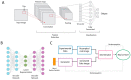
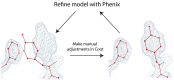
Single particle cryo-electron microscopy (cryo-EM) has emerged as the prevailing method for near-atomic structure determination, shedding light on the important molecular mechanisms of biological macromolecules. However, the inherent dynamics and structural variability of biological complexes coupled with the large number of experimental images generated by a cryo-EM experiment make data processing nontrivial. In particular, ab initio reconstruction and atomic model building remain major bottlenecks that demand substantial computational resources and manual intervention. Approaches utilizing recent innovations in artificial intelligence (AI) technology, particularly deep learning, have the potential to overcome the limitations that cannot be adequately addressed by traditional image processing approaches. Here, we review newly proposed AI-based methods for ab initio volume generation, heterogeneous 3D reconstruction, and atomic model building. We highlight the advancements made by the implementation of AI methods, as well as discuss remaining limitations and areas for future development.
Keywords: AI; AlphaFold2; artificial intelligence; cryo-EM; cryo-electron microscopy; deep learning; machine learning; neural networks.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Exploring the Structural Variability of Dynamic Biological Complexes by Single-Particle Cryo-Electron Microscopy.Micromachines (Basel). 2022 Dec 31;14(1):118. doi: 10.3390/mi14010118. Micromachines (Basel). 2022. PMID: 36677177 Free PMC article. Review.
-
Artificial Intelligence in Cryo-Electron Microscopy.Life (Basel). 2022 Aug 19;12(8):1267. doi: 10.3390/life12081267. Life (Basel). 2022. PMID: 36013446 Free PMC article. Review.
-
CryoVirusDB: A Labeled Cryo-EM Image Dataset for AI-Driven Virus Particle Picking.bioRxiv [Preprint]. 2023 Dec 26:2023.12.25.573312. doi: 10.1101/2023.12.25.573312. bioRxiv. 2023. PMID: 38234823 Free PMC article. Preprint.
-
Cryo2StructData: A Large Labeled Cryo-EM Density Map Dataset for AI-based Modeling of Protein Structures.bioRxiv [Preprint]. 2024 Jan 2:2023.06.14.545024. doi: 10.1101/2023.06.14.545024. bioRxiv. 2024. Update in: Sci Data. 2024 May 6;11(1):458. doi: 10.1038/s41597-024-03299-9. PMID: 37398020 Free PMC article. Updated. Preprint.
-
Revealing biomolecular structure and motion with neural ab initio cryo-EM reconstruction.bioRxiv [Preprint]. 2024 Jun 2:2024.05.30.596729. doi: 10.1101/2024.05.30.596729. bioRxiv. 2024. PMID: 38854058 Free PMC article. Preprint.
Cited by
-
Artificial intelligence and the analysis of cryo-EM data provide structural insight into the molecular mechanisms underlying LN-lamininopathies.Sci Rep. 2023 Oct 19;13(1):17825. doi: 10.1038/s41598-023-45200-5. Sci Rep. 2023. PMID: 37857770 Free PMC article.
-
The advent of preventive high-resolution structural histopathology by artificial-intelligence-powered cryogenic electron tomography.Front Mol Biosci. 2024 May 29;11:1390858. doi: 10.3389/fmolb.2024.1390858. eCollection 2024. Front Mol Biosci. 2024. PMID: 38868297 Free PMC article. Review.
-
Exploring the Structural Variability of Dynamic Biological Complexes by Single-Particle Cryo-Electron Microscopy.Micromachines (Basel). 2022 Dec 31;14(1):118. doi: 10.3390/mi14010118. Micromachines (Basel). 2022. PMID: 36677177 Free PMC article. Review.
-
Cryo-Electron Microscopy Studies of Biomolecular Structure and Dynamics.Micromachines (Basel). 2024 Aug 29;15(9):1092. doi: 10.3390/mi15091092. Micromachines (Basel). 2024. PMID: 39337752 Free PMC article.
-
De novo atomic protein structure modeling for cryoEM density maps using 3D transformer and HMM.Nat Commun. 2024 Jun 29;15(1):5511. doi: 10.1038/s41467-024-49647-6. Nat Commun. 2024. PMID: 38951555 Free PMC article.
References
-
- DiIorio M.C., Kulczyk A.W. A Robust Single-Particle Cryo-Electron Microscopy (cryo-EM) Processing Workflow with cryoSPARC, RELION, and Scipion. J. Vis. Exp. 2022;179:e63387. - PubMed
-
- Burley S.K., Berman H.M., Chiu W., Dai W., Flatt J.W., Hudson B.P., Kaelber J.T., Khare S.D., Kulczyk A.W., Lawson C.L., et al. Electron microscopy holdings of the Protein Data Bank: The impact of the resolution revolution, new validation tools, and implications for the future. Biophys. Rev. 2022;14:1281–1301. doi: 10.1007/s12551-022-01013-w. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

