Effects of Dibutylphthalate and Steroid Hormone Mixture on Human Prostate Cells
- PMID: 37762641
- PMCID: PMC10531810
- DOI: 10.3390/ijms241814341
Effects of Dibutylphthalate and Steroid Hormone Mixture on Human Prostate Cells
Abstract
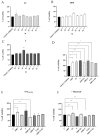
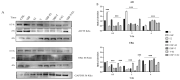
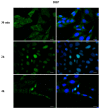
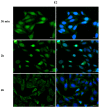
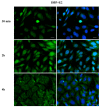
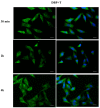
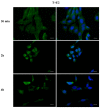
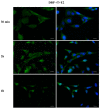
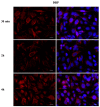
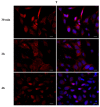
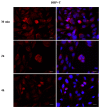
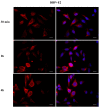
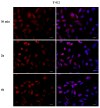
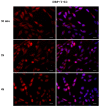
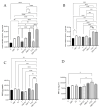
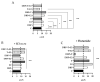
Phthalates are a family of aromatic chemical compounds mainly used as plasticizers. Among phthalates, di-n-butyl phthalate (DBP) is a low-molecular-weight phthalate used as a component of many cosmetic products, such as nail polish, and other perfumed personal care products. DBP has toxic effects on reproductive health, inducing testicular damage and developmental malformations. Inside the male reproductive system, the prostate gland reacts to both male and female sex steroids. For this reason, it represents an important target of endocrine-disrupting chemicals (EDCs), compounds that are able to affect the estrogen and androgen signaling pathways, thus interfering with prostate homeostasis and inducing several prostate pathologies. The aim of this project was to investigate the effects of DBP, alone and in combination with testosterone (T), 17β-estradiol (E2), and both, on the normal PNT1A human prostate cell-derived cell line, to mimic environmental contamination. We showed that DBP and all of the tested mixtures increase cell viability through activation of both estrogen receptor α (ERα) and androgen receptor (AR). DBP modulated steroid receptor levels in a nonmonotonic way, and differently to endogenous hormones. In addition, DBP translocated ERα to the nucleus over different durations and for a more prolonged time than E2, altering the normal responsiveness of prostate cells. However, DBP alone seemed not to influence AR localization, but AR was continuously and persistently activated when DBP was used in combination. Our results show that DBP alone, and in mixture, alters redox homeostasis in prostate cells, leading to a greater increase in cell oxidative susceptibility. In addition, we also demonstrate that DBP increases the migratory potential of PNT1A cells. In conclusion, our findings demonstrate that DBP, alone and in mixtures with endogenous steroid hormones, acts as an EDC, resulting in an altered prostate cell physiology and making these cells more prone to cancer transformation.
Keywords: androgens; di-n-butyl phthalate (DBP); endocrine-disrupting chemicals (EDCs); estrogens; phthalates; prostate gland; steroid receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Interference of dibutylphthalate on human prostate cell viability.Ecotoxicol Environ Saf. 2018 Jan;147:565-573. doi: 10.1016/j.ecoenv.2017.09.030. Epub 2017 Sep 15. Ecotoxicol Environ Saf. 2018. PMID: 28918339
-
Cell growth of BG-1 ovarian cancer cells is promoted by di-n-butyl phthalate and hexabromocyclododecane via upregulation of the cyclin D and cyclin-dependent kinase-4 genes.Mol Med Rep. 2012 Mar;5(3):761-6. doi: 10.3892/mmr.2011.712. Epub 2011 Dec 15. Mol Med Rep. 2012. PMID: 22179484
-
NTP technical report on the toxicity studies of Dibutyl Phthalate (CAS No. 84-74-2) Administered in Feed to F344/N Rats and B6C3F1 Mice.Toxic Rep Ser. 1995 Apr;30:1-G5. Toxic Rep Ser. 1995. PMID: 12209194
-
Impacts of endocrine-disrupting chemicals on prostate function and cancer.Environ Res. 2022 Mar;204(Pt B):112085. doi: 10.1016/j.envres.2021.112085. Epub 2021 Sep 22. Environ Res. 2022. PMID: 34562481 Review.
-
Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity.Int J Androl. 2010 Apr;33(2):443-62. doi: 10.1111/j.1365-2605.2009.01049.x. Int J Androl. 2010. PMID: 20487044 Free PMC article. Review.
Cited by
-
Juniperus oxycedrus L. ssp. Essential Oil Microneedles: A Promising Antimicrobial and Wound Healing Activity.Pharmaceuticals (Basel). 2023 Dec 27;17(1):40. doi: 10.3390/ph17010040. Pharmaceuticals (Basel). 2023. PMID: 38256874 Free PMC article.
-
Glyphosate Exposure Induces Cytotoxicity, Mitochondrial Dysfunction and Activation of ERα and ERβ Estrogen Receptors in Human Prostate PNT1A Cells.Int J Mol Sci. 2024 Jun 27;25(13):7039. doi: 10.3390/ijms25137039. Int J Mol Sci. 2024. PMID: 39000147 Free PMC article.
References
-
- Rodriguez-Sosa J.R., Ruiz S., Valdez D., Tullot T. Dibutyl phthalate affects the recovery, size, and viability of pig testicular tissue ectopically grafted in immunocompromised mice. FASEB J. 2020;34:1. doi: 10.1096/fasebj.2020.34.s1.07518. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

