Uncovering the Early Events Associated with Oligomeric Aβ-Induced Src Activation
- PMID: 37760073
- PMCID: PMC10525724
- DOI: 10.3390/antiox12091770
Uncovering the Early Events Associated with Oligomeric Aβ-Induced Src Activation
Abstract
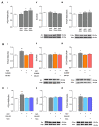
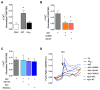
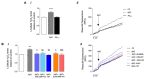
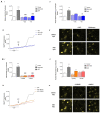
Soluble Aβ1-42 oligomers (AβO) are formed in the early stages of Alzheimer's disease (AD) and were previously shown to trigger enhanced Ca2+ levels and mitochondrial dysfunction via the activation of N-methyl-D-aspartate receptors (NMDAR). Src kinase is a ubiquitous redox-sensitive non-receptor tyrosine kinase involved in the regulation of several cellular processes, which was demonstrated to have a reciprocal interaction towards NMDAR activation. However, little is known about the early-stage mechanisms associated with AβO-induced neurodysfunction involving Src. Thus, in this work, we analysed the influence of brief exposure to oligomeric Aβ1-42 on Src activation and related mechanisms involving mitochondria and redox changes in mature primary rat hippocampal neurons. Data show that brief exposure to AβO induce H2O2-dependent Src activation involving different cellular events, including NMDAR activation and mediated intracellular Ca2+ rise, enhanced cytosolic and subsequent mitochondrial H2O2 levels, accompanied by mild mitochondrial fragmentation. Interestingly, these effects were prevented by Src inhibition, suggesting a feedforward modulation. The current study supports a relevant role for Src kinase activation in promoting the loss of postsynaptic glutamatergic synapse homeostasis involving cytosolic and mitochondrial ROS generation after brief exposure to AβO. Therefore, restoring Src activity can constitute a protective strategy for mitochondria and related hippocampal glutamatergic synapses.
Keywords: Alzheimer’s disease; NMDA receptor; Src tyrosine kinase; mitochondrial dysfunction; mitochondrial morphology.
Conflict of interest statement
The authors declare no competing financial interests or any conflicts of interest.
Figures
Similar articles
-
Amyloid β Oligomers Increase ER-Mitochondria Ca2+ Cross Talk in Young Hippocampal Neurons and Exacerbate Aging-Induced Intracellular Ca2+ Remodeling.Front Cell Neurosci. 2019 Feb 8;13:22. doi: 10.3389/fncel.2019.00022. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30800057 Free PMC article.
-
Aβ and NMDAR activation cause mitochondrial dysfunction involving ER calcium release.Neurobiol Aging. 2015 Feb;36(2):680-92. doi: 10.1016/j.neurobiolaging.2014.09.006. Epub 2014 Sep 6. Neurobiol Aging. 2015. PMID: 25442114
-
Endoplasmic reticulum stress occurs downstream of GluN2B subunit of N-methyl-d-aspartate receptor in mature hippocampal cultures treated with amyloid-β oligomers.Aging Cell. 2012 Oct;11(5):823-33. doi: 10.1111/j.1474-9726.2012.00848.x. Epub 2012 Jul 16. Aging Cell. 2012. PMID: 22708890
-
Remodeling of Intracellular Ca2+ Homeostasis in Rat Hippocampal Neurons Aged In Vitro.Int J Mol Sci. 2020 Feb 24;21(4):1549. doi: 10.3390/ijms21041549. Int J Mol Sci. 2020. PMID: 32102482 Free PMC article. Review.
-
Oligomeric Aβ-induced synaptic dysfunction in Alzheimer's disease.Mol Neurodegener. 2014 Nov 14;9:48. doi: 10.1186/1750-1326-9-48. Mol Neurodegener. 2014. PMID: 25394486 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

