Methods to assess COPD medications adherence in healthcare databases: a systematic review
- PMID: 37758274
- PMCID: PMC10523153
- DOI: 10.1183/16000617.0103-2023
Methods to assess COPD medications adherence in healthcare databases: a systematic review
Abstract
Background: The Global Initiative for Chronic Obstructive Lung Disease 2023 report recommends medication adherence assessment in COPD as an action item. Healthcare databases provide opportunities for objective assessments; however, multiple methods exist. We aimed to systematically review the literature to describe existing methods to assess adherence in COPD in healthcare databases and to evaluate the reporting of influencing variables.
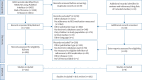
Method: We searched MEDLINE, Web of Science and Embase for peer-reviewed articles evaluating adherence to COPD medication in electronic databases, written in English, published up to 11 October 2022 (PROSPERO identifier CRD42022363449). Two reviewers independently conducted screening for inclusion and performed data extraction. Methods to assess initiation (dispensing of medication after prescribing), implementation (extent of use over a specific time period) and/or persistence (time from initiation to discontinuation) were listed descriptively. Each included study was evaluated for reporting variables with an impact on adherence assessment: inpatient stays, drug substitution, dose switching and early refills.
Results: 160 studies were included, of which four assessed initiation, 135 implementation and 45 persistence. Overall, one method was used to measure initiation, 43 methods for implementation and seven methods for persistence. Most of the included implementation studies reported medication possession ratio, proportion of days covered and/or an alteration of these methods. Only 11% of the included studies mentioned the potential impact of the evaluated variables.
Conclusion: Variations in adherence assessment methods are common. Attention to transparency, reporting of variables with an impact on adherence assessment and rationale for choosing an adherence cut-off or treatment gap is recommended.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: Outside this manuscript, J.K. Quint received grants paid to her institution from the Medical Research Council, Health Data Research UK, GlaxoSmithKline, Boehringer Ingelheim, Asthma + Lung UK and AstraZeneca, and consulting fees from GlaxoSmithKline, Evidera, AstraZeneca and Insmed. None of which are related to the content of this work. Outside this manuscript, K. Verhamme received unconditional research grants paid to her institution from Chiesi, Amgen, Union Chimique Belge (UCB), Johnson & Johnson (J&J) and the European Medicines Agency (EMA). None of which are related to the content of this work. Outside this manuscript, L. Lahousse received a consulting fee paid to her institution from AstraZeneca and honoraria for lectures paid to her institution from Chiesi and IPSA vzw, a non-profit organisation facilitating lifelong learning for healthcare providers. L. Lahousse is an unpaid member of European Respiratory Society and Belgian Respiratory Society, member of Faculty board of Ghent University – Faculty of Pharmaceutical Sciences and faculty committees. None of which are related to the content of this work. All other authors declare no competing interests.
Figures
Similar articles
-
Medication adherence to inhalation therapy and the risk of COPD exacerbations: a systematic review with meta-analysis.BMJ Open Respir Res. 2024 Sep 19;11(1):e001964. doi: 10.1136/bmjresp-2023-001964. BMJ Open Respir Res. 2024. PMID: 39304207 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Characteristics of patients with COPD newly prescribed a long-acting bronchodilator: a retrospective cohort study.Int J Chron Obstruct Pulmon Dis. 2014 Sep 25;9:1021-31. doi: 10.2147/COPD.S58258. eCollection 2014. Int J Chron Obstruct Pulmon Dis. 2014. PMID: 25285002 Free PMC article.
-
Adherence to controller therapy for chronic obstructive pulmonary disease: a review.Curr Med Res Opin. 2010 Oct;26(10):2421-9. doi: 10.1185/03007995.2010.516284. Curr Med Res Opin. 2010. PMID: 20815661 Review.
-
Medication (re)fill adherence measures derived from pharmacy claims data in older Americans: a review of the literature.Drugs Aging. 2013 Jun;30(6):383-99. doi: 10.1007/s40266-013-0074-z. Drugs Aging. 2013. PMID: 23553512 Review.
Cited by
-
Potential marker genes for chronic obstructive pulmonary disease revealed based on single-cell sequencing and Mendelian randomization analysis.Aging (Albany NY). 2024 May 23;16(10):8922-8943. doi: 10.18632/aging.205849. Epub 2024 May 23. Aging (Albany NY). 2024. PMID: 38787375 Free PMC article.
-
Medication adherence to inhalation therapy and the risk of COPD exacerbations: a systematic review with meta-analysis.BMJ Open Respir Res. 2024 Sep 19;11(1):e001964. doi: 10.1136/bmjresp-2023-001964. BMJ Open Respir Res. 2024. PMID: 39304207 Free PMC article.
References
-
- World Health Organization (WHO) . The Top 10 Causes of Death. www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Date last updated: 9 December 2020. Date last accessed: 9 May 2023.
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global Strategy for Prevention, Diagnosis and Management of COPD: 2023 Report. https://goldcopd.org/2023-gold-report-2/. Date last updated: 14 November 2022. Date last accessed: 5 January 2023.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous