Paracoccidioides brasiliensis Induces α3 Integrin Lysosomal Degradation in Lung Epithelial Cells
- PMID: 37755020
- PMCID: PMC10532483
- DOI: 10.3390/jof9090912
Paracoccidioides brasiliensis Induces α3 Integrin Lysosomal Degradation in Lung Epithelial Cells
Abstract
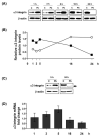
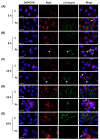
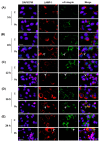
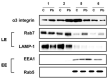
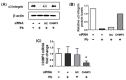
Studies on the pathogen-host interaction are crucial for the understanding of the mechanisms involved in the establishment, maintenance, and spread of infection. In recent years, our research group has observed that the P. brasiliensis species interact with integrin family receptors and increase the expression of α3 integrin in lung epithelial cells within 5 h of infection. Interestingly, α3 integrin levels were reduced by approximately 99% after 24 h of infection with P. brasiliensis compared to non-infected cells. In this work, we show that, during infection with this fungus, α3 integrin is increased in the late endosomes of A549 lung epithelial cells. We also observed that the inhibitor of the lysosomal activity bafilomycin A1 was able to inhibit the decrease in α3 integrin levels. In addition, the silencing of the charged multivesicular body protein 3 (CHMP3) inhibited the reduction in α3 integrin levels induced by P. brasiliensis in A549 cells. Thus, together, these results indicate that this fungus induces the degradation of α3 integrin in A549 lung epithelial cells by hijacking the host cell endolysosomal pathway.
Keywords: Paracoccidioides brasiliensis; epithelial cell; lysosomal degradation; vesicular traffic; α3 integrin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Paracoccidioides brasiliensis downmodulates α3 integrin levels in human lung epithelial cells in a TLR2-dependent manner.Sci Rep. 2020 Nov 10;10(1):19483. doi: 10.1038/s41598-020-76557-6. Sci Rep. 2020. PMID: 33173103 Free PMC article.
-
Paracoccidioides brasiliensis induces recruitment of α3 and α5 integrins into epithelial cell membrane rafts, leading to cytokine secretion.Microbes Infect. 2016 Jan;18(1):68-77. doi: 10.1016/j.micinf.2015.09.003. Epub 2015 Sep 11. Microbes Infect. 2016. PMID: 26369712
-
Paracoccidioides species present distinct fungal adherence to epithelial lung cells and promote different IL-8 secretion levels.Med Microbiol Immunol. 2020 Feb;209(1):59-67. doi: 10.1007/s00430-019-00639-0. Epub 2019 Oct 31. Med Microbiol Immunol. 2020. PMID: 31673845
-
Paracoccidioides brasiliensis induces secretion of IL-6 and IL-8 by lung epithelial cells. Modulation of host cytokine levels by fungal proteases.Microbes Infect. 2012 Oct;14(12):1077-85. doi: 10.1016/j.micinf.2012.05.016. Epub 2012 Jun 9. Microbes Infect. 2012. PMID: 22687715
-
Interactions of Paracoccidioides brasiliensis with host cells: recent advances.Mycopathologia. 2008 Apr-May;165(4-5):237-48. doi: 10.1007/s11046-007-9074-z. Mycopathologia. 2008. PMID: 17940851 Review.
References
-
- Shikanai-Yasuda M.A., Mendes R.P., Colombo A.L., de Queiroz Telles F., Kono A., Paniago A.M.M., Nathan A., do Valle A.C.F., Bagagli E., Benard G., et al. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Epidemiol. Serv. Saude. 2018;27:e0500001. doi: 10.1590/0037-8682-0230-2017. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

