Nuclear Factor-Kappa B Regulation of Osteoclastogenesis and Osteoblastogenesis
- PMID: 37749800
- PMCID: PMC10613774
- DOI: 10.3803/EnM.2023.501
Nuclear Factor-Kappa B Regulation of Osteoclastogenesis and Osteoblastogenesis
Abstract
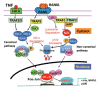
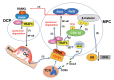
Maintenance of skeletal integrity requires the coordinated activity of multinucleated bone-resorbing osteoclasts and bone-forming osteoblasts. Osteoclasts form resorption lacunae on bone surfaces in response to cytokines by fusion of precursor cells. Osteoblasts are derived from mesenchymal precursors and lay down new bone in resorption lacunae during bone remodeling. Nuclear factorkappa B (NF-κB) signaling regulates osteoclast and osteoblast formation and is activated in osteoclast precursors in response to the essential osteoclastogenic cytokine, receptor activator of NF-κB ligand (RANKL), which can also control osteoblast formation through RANK-RANKL reverse signaling in osteoblast precursors. RANKL and some pro-inflammatory cytokines, including tumor necrosis factor (TNF), activate NF-κB signaling to positively regulate osteoclast formation and functions. However, these cytokines also limit osteoclast and osteoblast formation through NF-κB signaling molecules, including TNF receptor-associated factors (TRAFs). TRAF6 mediates RANKL-induced osteoclast formation through canonical NF-κB signaling. In contrast, TRAF3 limits RANKL- and TNF-induced osteoclast formation, and it restricts transforming growth factor β (TGFβ)-induced inhibition of osteoblast formation in young and adult mice. During aging, neutrophils expressing TGFβ and C-C chemokine receptor type 5 (CCR5) increase in bone marrow of mice in response to increased NF-κB-induced CC motif chemokine ligand 5 (CCL5) expression by mesenchymal progenitor cells and injection of these neutrophils into young mice decreased bone mass. TGFβ causes degradation of TRAF3, resulting in decreased glycogen synthase kinase-3β/β-catenin-mediated osteoblast formation and age-related osteoporosis in mice. The CCR5 inhibitor, maraviroc, prevented accumulation of TGFβ+/CCR5+ neutrophils in bone marrow and increased bone mass by inhibiting bone resorption and increasing bone formation in aged mice. This paper updates current understanding of how NF-κB signaling is involved in the positive and negative regulation of cytokine-mediated osteoclast and osteoblast formation and activation with a focus on the role of TRAF3 signaling, which can be targeted therapeutically to enhance bone mass.
Keywords: Aging; Osteoblasts; Osteoclasts; Osteoporosis; TNF receptor-associated factor 3; TNF receptor-associated factor 6; Transforming growth factor-beta.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

Similar articles
-
Bone Remodeling and the Role of TRAF3 in Osteoclastic Bone Resorption.Front Immunol. 2018 Sep 28;9:2263. doi: 10.3389/fimmu.2018.02263. eCollection 2018. Front Immunol. 2018. PMID: 30323820 Free PMC article. Review.
-
RANKL cytokine enhances TNF-induced osteoclastogenesis independently of TNF receptor associated factor (TRAF) 6 by degrading TRAF3 in osteoclast precursors.J Biol Chem. 2017 Jun 16;292(24):10169-10179. doi: 10.1074/jbc.M116.771816. Epub 2017 Apr 24. J Biol Chem. 2017. PMID: 28438834 Free PMC article.
-
Exogenous regucalcin stimulates osteoclastogenesis and suppresses osteoblastogenesis through NF-κB activation.Mol Cell Biochem. 2012 Jan;359(1-2):193-203. doi: 10.1007/s11010-011-1014-z. Epub 2011 Aug 14. Mol Cell Biochem. 2012. PMID: 21842421
-
TGFβ-induced degradation of TRAF3 in mesenchymal progenitor cells causes age-related osteoporosis.Nat Commun. 2019 Jun 26;10(1):2795. doi: 10.1038/s41467-019-10677-0. Nat Commun. 2019. PMID: 31243287 Free PMC article.
-
NF-κB-Mediated Regulation of Osteoclastogenesis.Endocrinol Metab (Seoul). 2015 Mar 27;30(1):35-44. doi: 10.3803/EnM.2015.30.1.35. Endocrinol Metab (Seoul). 2015. PMID: 25827455 Free PMC article. Review.
Cited by
-
Genetic Deficiency of the Long Pentraxin 3 Affects Osteogenesis and Osteoclastogenesis in Homeostatic and Inflammatory Conditions.Int J Mol Sci. 2023 Nov 23;24(23):16648. doi: 10.3390/ijms242316648. Int J Mol Sci. 2023. PMID: 38068970 Free PMC article.
-
Effect of recombinant human bone morphogenetic protein-2 and osteoprotegerin-Fc in MC3T3-E1 cells: beyond challenges to success.J Rheum Dis. 2024 Jul 1;31(3):133-134. doi: 10.4078/jrd.2024.0079. J Rheum Dis. 2024. PMID: 38957364 Free PMC article. No abstract available.
-
Evaluation of Immunohistochemical Biomarkers in Diabetic Wistar Rats with Periodontal Disease.J Pers Med. 2024 May 15;14(5):527. doi: 10.3390/jpm14050527. J Pers Med. 2024. PMID: 38793109 Free PMC article.
-
Promotion of Bone Formation in a Rat Osteoporotic Vertebral Body Defect Model via Suppression of Osteoclastogenesis by Ectopic Embryonic Calvaria Derived Mesenchymal Stem Cells.Int J Mol Sci. 2024 Jul 26;25(15):8174. doi: 10.3390/ijms25158174. Int J Mol Sci. 2024. PMID: 39125746 Free PMC article.
-
The Role of Rosavin in the Pathophysiology of Bone Metabolism.Int J Mol Sci. 2024 Feb 9;25(4):2117. doi: 10.3390/ijms25042117. Int J Mol Sci. 2024. PMID: 38396794 Free PMC article. Review.
References
-
- Tsukasaki M, Huynh NC, Okamoto K, Muro R, Terashima A, Kurikawa Y, et al. Stepwise cell fate decision pathways during osteoclastogenesis at single-cell resolution. Nat Metab. 2020;2:1382–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

