Molecular mechanisms of inorganic-phosphate release from the core and barbed end of actin filaments
- PMID: 37749275
- PMCID: PMC10643162
- DOI: 10.1038/s41594-023-01101-9
Molecular mechanisms of inorganic-phosphate release from the core and barbed end of actin filaments
Abstract
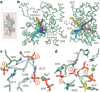
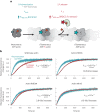
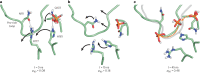
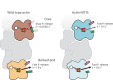
The release of inorganic phosphate (Pi) from actin filaments constitutes a key step in their regulated turnover, which is fundamental to many cellular functions. The mechanisms underlying Pi release from the core and barbed end of actin filaments remain unclear. Here, using human and bovine actin isoforms, we combine cryo-EM with molecular-dynamics simulations and in vitro reconstitution to demonstrate how actin releases Pi through a 'molecular backdoor'. While constantly open at the barbed end, the backdoor is predominantly closed in filament-core subunits and opens only transiently through concerted amino acid rearrangements. This explains why Pi escapes rapidly from the filament end but slowly from internal subunits. In a nemaline-myopathy-associated actin variant, the backdoor is predominantly open in filament-core subunits, resulting in accelerated Pi release and filaments with drastically shortened ADP-Pi caps. Our results provide the molecular basis for Pi release from actin and exemplify how a disease-linked mutation distorts the nucleotide-state distribution and atomic structure of the filament.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures










Similar articles
-
High microfilament concentration results in barbed-end ADP caps.Biophys J. 1993 Nov;65(5):1757-66. doi: 10.1016/S0006-3495(93)81271-2. Biophys J. 1993. PMID: 8298009 Free PMC article.
-
Multicomponent depolymerization of actin filament pointed ends by cofilin and cyclase-associated protein depends upon filament age.Eur J Cell Biol. 2024 Jun;103(2):151423. doi: 10.1016/j.ejcb.2024.151423. Epub 2024 May 22. Eur J Cell Biol. 2024. PMID: 38796920
-
A change in actin conformation associated with filament instability after Pi release.Proc Natl Acad Sci U S A. 1999 Jan 5;96(1):29-34. doi: 10.1073/pnas.96.1.29. Proc Natl Acad Sci U S A. 1999. PMID: 9874766 Free PMC article.
-
Regulators of actin filament barbed ends at a glance.J Cell Sci. 2016 Mar 15;129(6):1085-91. doi: 10.1242/jcs.179994. Epub 2016 Mar 3. J Cell Sci. 2016. PMID: 26940918 Review.
-
Actin polymerization and ATP hydrolysis.Adv Biophys. 1990;26:51-73. doi: 10.1016/0065-227x(90)90007-g. Adv Biophys. 1990. PMID: 2082729 Review.
Cited by
-
Mechanism of phosphate release from actin filaments.Proc Natl Acad Sci U S A. 2024 Jul 16;121(29):e2408156121. doi: 10.1073/pnas.2408156121. Epub 2024 Jul 9. Proc Natl Acad Sci U S A. 2024. PMID: 38980907 Free PMC article.
-
Phalloidin and DNase I-bound F-actin pointed end structures reveal principles of filament stabilization and disassembly.Nat Commun. 2024 Sep 11;15(1):7969. doi: 10.1038/s41467-024-52251-3. Nat Commun. 2024. PMID: 39261469 Free PMC article.
-
Helical reconstruction, again.Curr Opin Struct Biol. 2024 Apr;85:102788. doi: 10.1016/j.sbi.2024.102788. Epub 2024 Feb 23. Curr Opin Struct Biol. 2024. PMID: 38401399 Review.
-
Cyclase-associated protein interacts with actin filament barbed ends to promote depolymerization and formin displacement.J Biol Chem. 2023 Dec;299(12):105367. doi: 10.1016/j.jbc.2023.105367. Epub 2023 Oct 19. J Biol Chem. 2023. PMID: 37863260 Free PMC article.
-
Mechanism of Phosphate Release from Actin Filaments.bioRxiv [Preprint]. 2024 May 12:2023.08.03.551904. doi: 10.1101/2023.08.03.551904. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2024 Jul 16;121(29):e2408156121. doi: 10.1073/pnas.2408156121. PMID: 37577500 Free PMC article. Updated. Preprint.
References
-
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments review. Cell. 2003;112:453–465. - PubMed
-
- Le Clainche C, Carlier MF. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol. Rev. 2008;88:489–513. - PubMed
-
- Rould MA, Wan Q, Joel PB, Lowey S, Trybus KM. Crystal structures of expressed non-polymerizable monomeric actin in the ADP and ATP states. J. Biol. Chem. 2006;281:31909–31919. - PubMed
-
- Oda T, Iwasa M, Aihara T, Maéda Y, Narita A. The nature of the globular- to fibrous-actin transition. Nature. 2009;457:441–445. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

