Uracil-DNA glycosylase of murine gammaherpesvirus 68 binds cognate viral replication factors independently of its catalytic residues
- PMID: 37747202
- PMCID: PMC10597349
- DOI: 10.1128/msphere.00278-23
Uracil-DNA glycosylase of murine gammaherpesvirus 68 binds cognate viral replication factors independently of its catalytic residues
Abstract
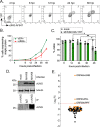
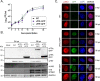
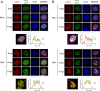
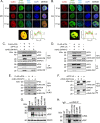
Herpesviruses are large double-stranded DNA viruses that encode core replication proteins and accessory factors involved in nucleotide metabolism and DNA repair. Mammalian uracil-DNA glycosylases (UNG) excise deleterious uracil residues from their genomic DNA. Each herpesvirus UNG studied to date has demonstrated conservation of the enzymatic function to excise uracil residues from DNA. We previously reported that a murine gammaherpesvirus (MHV68) with a stop codon in ORF46 (ORF46.stop) that encodes for vUNG was defective in lytic replication and latency in vivo. However, a mutant virus that expressed a catalytically inactive vUNG (ORF46.CM) had no replication defect unless coupled with additional mutations in the catalytic motif of the viral dUTPase (ORF54.CM). The disparate phenotypes observed in the vUNG mutants led us to explore the non-enzymatic properties of vUNG. Immunoprecipitation of vUNG followed by mass spectrometry in MHV68-infected fibroblasts identified a complex comprising the cognate viral DNA polymerase, vPOL, encoded by ORF9, and the viral DNA polymerase processivity factor, vPPF, encoded by ORF59. MHV68 vUNG co-localized with vPOL and vPPF in subnuclear structures consistent with viral replication compartments. In reciprocal co-immunoprecipitations, the vUNG formed a complex with the vPOL and vPPF upon transfection with either factor alone or in combination. Lastly, we determined that key catalytic residues of vUNG are not required for interactions with vPOL and vPPF upon transfection or in the context of infection. We conclude that the vUNG of MHV68 associates with vPOL and vPPF independently of its catalytic activity. IMPORTANCE Gammaherpesviruses encode a uracil-DNA glycosylase (vUNG) that is presumed to excise uracil residues from viral genomes. We previously identified the vUNG enzymatic activity, but not the protein itself, as dispensable for gammaherpesvirus replication in vivo. In this study, we report a non-enzymatic role for the viral UNG of a murine gammaherpesvirus in forming a complex with two key components of the viral DNA replication machinery. Understanding the role of the vUNG in this viral DNA replication complex may inform the development of antiviral drugs that combat gammaherpesvirus-associated cancers.
Keywords: gammaherpesvirus; lytic replication; uracil-DNA glycosylase; viral DNA polymerase; viral DNA polymerase processivity factor; viral replication compartment; virus-host interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Update of
-
Uracil-DNA Glycosylase of Murine Gammaherpesvirus 68 Binds Cognate Viral Replication Factors Independently of its Catalytic Residues.bioRxiv [Preprint]. 2023 May 19:2023.05.19.541466. doi: 10.1101/2023.05.19.541466. bioRxiv. 2023. Update in: mSphere. 2023 Oct 24;8(5):e0027823. doi: 10.1128/msphere.00278-23. PMID: 37398059 Free PMC article. Updated. Preprint.
Similar articles
-
Uracil-DNA Glycosylase of Murine Gammaherpesvirus 68 Binds Cognate Viral Replication Factors Independently of its Catalytic Residues.bioRxiv [Preprint]. 2023 May 19:2023.05.19.541466. doi: 10.1101/2023.05.19.541466. bioRxiv. 2023. Update in: mSphere. 2023 Oct 24;8(5):e0027823. doi: 10.1128/msphere.00278-23. PMID: 37398059 Free PMC article. Updated. Preprint.
-
Combinatorial Loss of the Enzymatic Activities of Viral Uracil-DNA Glycosylase and Viral dUTPase Impairs Murine Gammaherpesvirus Pathogenesis and Leads to Increased Recombination-Based Deletion in the Viral Genome.mBio. 2018 Oct 30;9(5):e01831-18. doi: 10.1128/mBio.01831-18. mBio. 2018. PMID: 30377280 Free PMC article.
-
Absence of the uracil DNA glycosylase of murine gammaherpesvirus 68 impairs replication and delays the establishment of latency in vivo.J Virol. 2015 Mar;89(6):3366-79. doi: 10.1128/JVI.03111-14. Epub 2015 Jan 14. J Virol. 2015. PMID: 25589640 Free PMC article.
-
Poxvirus uracil-DNA glycosylase-An unusual member of the family I uracil-DNA glycosylases.Protein Sci. 2016 Dec;25(12):2113-2131. doi: 10.1002/pro.3058. Epub 2016 Nov 2. Protein Sci. 2016. PMID: 27684934 Free PMC article. Review.
-
The vaccinia virus DNA polymerase and its processivity factor.Virus Res. 2017 Apr 15;234:193-206. doi: 10.1016/j.virusres.2017.01.027. Epub 2017 Feb 1. Virus Res. 2017. PMID: 28159613 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

