BiCl3-catalyzed green synthesis of 4-hydroxy-2-quinolone analogues under microwave irradiation
- PMID: 37746335
- PMCID: PMC10517106
- DOI: 10.1039/d3ra05289c
BiCl3-catalyzed green synthesis of 4-hydroxy-2-quinolone analogues under microwave irradiation
Abstract
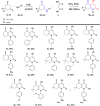
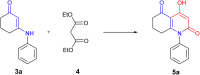
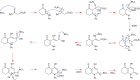
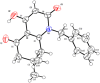
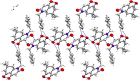
Traditional chemical synthesis, which involves the use of dangerous protocols, hazardous solvents, and toxic products and catalysts, is considered environmentally inappropriate and harmful to human health. Bearing in mind its numerous drawbacks, it has become crucial to substitute conventional chemistry with green chemistry which is safer, more ecofriendly and more effective in terms of time and selectivity. Elaborating synthetic protocols producing interesting new compounds using both microwave heating and heterogeneous non-toxic catalysts is acknowledged as a green approach that avoids many classical chemistry-related problems. In the current study, β-enaminones were used as precursors to the synthesis of modified 4-hydroxy-2-quinolone analogues. The synthesis was monitored in a benign way under microwave irradiation and was catalyzed by bismuth chloride III in an amount of 20 mol%. This method is privileged by using a non-corrosive, non-toxic, low-cost and available bismuth Lewis acid catalyst that has made it more respectful to the demands of green chemistry. The synthesized compounds were obtained in moderate to good yields (51-71%) and were characterized by 1H, 13C NMR, and IR spectroscopy as well as elemental analysis. Compound 5i was subjected to a complete structural elucidation using the X-ray diffraction method, and the results show the obtention of the enolic tautomeric form.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
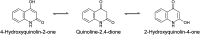

Similar articles
-
Green Synthesis of Fused Chromeno-pyrazolo-phthalazine Derivatives with Silicasupported Bismuth Nitrate under Solvent-free Conditions.Curr Org Synth. 2021;18(8):854-861. doi: 10.2174/1570179417666201208112520. Curr Org Synth. 2021. PMID: 33292122
-
Synthesis of Medicinally Privileged Heterocycles through Dielectric Heating.Curr Med Chem. 2017;24(41):4596-4626. doi: 10.2174/0929867324666170223152137. Curr Med Chem. 2017. PMID: 28240166 Review.
-
Citrus Juice: Green and Natural Catalyst for the Solvent-free Silica Supported Synthesis of β-Enaminones Using Grindstone Technique.Comb Chem High Throughput Screen. 2018;21(1):19-25. doi: 10.2174/1386207321666180102115733. Comb Chem High Throughput Screen. 2018. PMID: 29295688
-
Microwave-induced Bismuth Salts-mediated Synthesis of Molecules of Medicinal Interests.Curr Med Chem. 2017;24(41):4677-4713. doi: 10.2174/0929867324666170320121142. Curr Med Chem. 2017. PMID: 28322155 Review.
-
Green Synthetic Approach: An Efficient Eco-Friendly Tool for Synthesis of Biologically Active Oxadiazole Derivatives.Molecules. 2021 Feb 22;26(4):1163. doi: 10.3390/molecules26041163. Molecules. 2021. PMID: 33671751 Free PMC article. Review.
References
-
- Henary M. Kananda C. Rotolo L. Savino B. Owens E. A. Cravotto G. Benefits and applications of microwave-assisted synthesis of nitrogen containing heterocycles in medicinal chemistry. RSC Adv. 2020;10:14170. - PMC - PubMed
- Adhikari A. Bhakta S. Ghosh T. Microwave-assisted synthesis of bioactive heterocycles: An overview. Tetrahedron. 2022;126:133085.
- Pham E. C. Truong T. N. Design, Microwave-Assisted Synthesis, Antimicrobial and Anticancer Evaluation, and In Silico Studies of Some 2-Naphthamide Derivatives as DHFR and VEGFR-2 Inhibitors. ACS Omega. 2022;7:33614. - PMC - PubMed
- Driowya M. Saber A. Marzag H. Demange L. Benhida R. Bougrin K. Microwave-Assisted Synthesis of Bioactive Six-Membered Heterocycles and Their Fused Analogues. Molecules. 2016;21:492. - PMC - PubMed
- Yadav D. K. Kaushik P. Pankaj Rana V. S. Kamil D. Khatri D. Shakil N. A. Microwave Assisted Synthesis, Characterization and Biological Activities of Ferrocenyl Chalcones and Their QSAR Analysis. Front. Chem. 2019;7:814. - PMC - PubMed
- Santagada V. Frecentese F. Perissutti E. Fiorino F. Severino B. Caliendo G. Microwave Assisted Synthesis: A New Technology in Drug Discovery. Mini-Rev. Med. Chem. 2009;9:1389. - PubMed
-
- Kappe C. O. Controlled microwave heating in modern organic synthesis. Angew Chem. Int. Ed. Engl. 2004;43:6250. - PubMed
-
- Laskar K. Bhattacharjee P. Gohain M. Deka D. Bora U. Application of bio-based green heterogeneous catalyst for the synthesis of arylidinemalononitriles. Sustainable Chem. Pharm. 2019;14:100181.
-
- Bhuyan P. Bhorali P. Kataky S. Bharali S. J. Guha A. K. Saikia L. ZnCl2 catalyzed cascade conjugative alkynylation/6-endo-dig cyclisation of N,N-dimethyl barbituric acid derived alkenes under ultrasonic irradiation: An improved, base & column-free access to pyrano[2,3-d]pyrimidine-2,4(3H,5H)-diones. Sustainable Chem. Pharm. 2022;30:100852.
-
- Esmaeilpour M. Javidi J. Zandi M. One-pot synthesis of multisubstituted imidazoles under solvent-free conditions and microwave irradiation using Fe3O4@SiO2–imid–PMAn magnetic porous nanospheres as a recyclable catalyst. New J. Chem. 2015;39:3388.
LinkOut - more resources
Full Text Sources

