Aminobisphosphonates reactivate the latent reservoir in people living with HIV-1
- PMID: 37744358
- PMCID: PMC10516574
- DOI: 10.3389/fimmu.2023.1219250
Aminobisphosphonates reactivate the latent reservoir in people living with HIV-1
Abstract
Antiretroviral therapy (ART) is not curative due to the existence of cellular reservoirs of latent HIV-1 that persist during therapy. Current research efforts to cure HIV-1 infection include "shock and kill" strategies to disrupt latency using small molecules or latency-reversing agents (LRAs) to induce expression of HIV-1 enabling cytotoxic immune cells to eliminate infected cells. The modest success of current LRAs urges the field to identify novel drugs with increased clinical efficacy. Aminobisphosphonates (N-BPs) that include pamidronate, zoledronate, or alendronate, are the first-line treatment of bone-related diseases including osteoporosis and bone malignancies. Here, we show the use of N-BPs as a novel class of LRA: we found in ex vivo assays using primary cells from ART-suppressed people living with HIV-1 that N-BPs induce HIV-1 from latency to levels that are comparable to the T cell activator phytohemagglutinin (PHA). RNA sequencing and mechanistic data suggested that reactivation may occur through activation of the activator protein 1 signaling pathway. Stored samples from a prior clinical trial aimed at analyzing the effect of alendronate on bone mineral density, provided further evidence of alendronate-mediated latency reversal and activation of immune effector cells. Decay of the reservoir measured by IPDA was however not detected. Our results demonstrate the novel use of N-BPs to reverse HIV-1 latency while inducing immune effector functions. This preliminary evidence merits further investigation in a controlled clinical setting possibly in combination with therapeutic vaccination.
Keywords: HIV cure; IPDA; aminobisphosphonates; gamma delta (γδ) T cells; latency reversing agents; shock and kill.
Copyright © 2023 Sanz, Weideman, Ward, Clohosey, Garcia-Recio, Selitsky, Mann, Iannone, Whitworth, Chitrakar, Garrido, Kirchherr, Coffey, Tsai, Samir, Xu, Copertino, Bosque, Jones, Parker, Hudgens, Goonetilleke and Soriano-Sarabia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


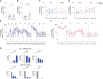
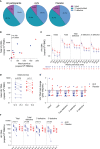
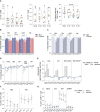
Update of
-
Aminobisphosphonates reactivate the latent reservoir in people living with HIV-1.bioRxiv [Preprint]. 2023 Feb 7:2023.02.07.527421. doi: 10.1101/2023.02.07.527421. bioRxiv. 2023. Update in: Front Immunol. 2023 Sep 06;14:1219250. doi: 10.3389/fimmu.2023.1219250 PMID: 36798291 Free PMC article. Updated. Preprint.
Similar articles
-
Aminobisphosphonates reactivate the latent reservoir in people living with HIV-1.bioRxiv [Preprint]. 2023 Feb 7:2023.02.07.527421. doi: 10.1101/2023.02.07.527421. bioRxiv. 2023. Update in: Front Immunol. 2023 Sep 06;14:1219250. doi: 10.3389/fimmu.2023.1219250 PMID: 36798291 Free PMC article. Updated. Preprint.
-
The HIV Latency Reversal Agent HODHBt Enhances NK Cell Effector and Memory-Like Functions by Increasing Interleukin-15-Mediated STAT Activation.J Virol. 2022 Aug 10;96(15):e0037222. doi: 10.1128/jvi.00372-22. Epub 2022 Jul 14. J Virol. 2022. PMID: 35867565 Free PMC article.
-
Alternate NF-κB-Independent Signaling Reactivation of Latent HIV-1 Provirus.J Virol. 2019 Aug 28;93(18):e00495-19. doi: 10.1128/JVI.00495-19. Print 2019 Sep 15. J Virol. 2019. PMID: 31243131 Free PMC article.
-
Shocking HIV-1 with immunomodulatory latency reversing agents.Semin Immunol. 2021 Jan;51:101478. doi: 10.1016/j.smim.2021.101478. Epub 2021 May 8. Semin Immunol. 2021. PMID: 33972164 Review.
-
Targeting Cellular and Tissue HIV Reservoirs With Toll-Like Receptor Agonists.Front Immunol. 2019 Oct 15;10:2450. doi: 10.3389/fimmu.2019.02450. eCollection 2019. Front Immunol. 2019. PMID: 31681325 Free PMC article. Review.
Cited by
-
Dual role of circulating and mucosal Vδ1 T cells in the control of and contribution to persistent HIV-1 infection.Res Sq [Preprint]. 2024 Aug 2:rs.3.rs-4784403. doi: 10.21203/rs.3.rs-4784403/v1. Res Sq. 2024. PMID: 39149467 Free PMC article. Preprint.
-
Interventions during Early Infection: Opening a Window for an HIV Cure?Viruses. 2024 Oct 9;16(10):1588. doi: 10.3390/v16101588. Viruses. 2024. PMID: 39459922 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

