Real-time in vivo thoracic spinal glutamate sensing during myocardial ischemia
- PMID: 37737733
- PMCID: PMC10908408
- DOI: 10.1152/ajpheart.00299.2023
Real-time in vivo thoracic spinal glutamate sensing during myocardial ischemia
Abstract
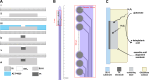
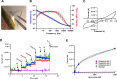
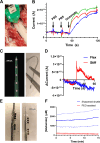
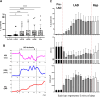
In the spinal cord, glutamate serves as the primary excitatory neurotransmitter. Monitoring spinal glutamate concentrations offers valuable insights into spinal neural processing. Consequently, spinal glutamate concentration has the potential to emerge as a useful biomarker for conditions characterized by increased spinal neural network activity, especially when uptake systems become dysfunctional. In this study, we developed a multichannel custom-made flexible glutamate-sensing probe for the large-animal model that is capable of measuring extracellular glutamate concentrations in real time and in vivo. We assessed the probe's sensitivity and specificity through in vitro and ex vivo experiments. Remarkably, this developed probe demonstrates nearly instantaneous glutamate detection and allows continuous monitoring of glutamate concentrations. Furthermore, we evaluated the mechanical and sensing performance of the probe in vivo, within the pig spinal cord. Moreover, we applied the glutamate-sensing method using the flexible probe in the context of myocardial ischemia-reperfusion (I/R) injury. During I/R injury, cardiac sensory neurons in the dorsal root ganglion transmit excitatory signals to the spinal cord, resulting in sympathetic activation that potentially leads to fatal arrhythmias. We have successfully shown that our developed glutamate-sensing method can detect this spinal network excitation during myocardial ischemia. This study illustrates a novel technique for measuring spinal glutamate at different spinal cord levels as a surrogate for the spinal neural network activity during cardiac interventions that engage the cardio-spinal neural pathway.NEW & NOTEWORTHY In this study, we have developed a new flexible sensing probe to perform an in vivo measurement of spinal glutamate signaling in a large animal model. Our initial investigations involved precise testing of this probe in both in vitro and ex vivo environments. We accurately assessed the sensitivity and specificity of our glutamate-sensing probe and demonstrated its performance. We also evaluated the performance of our developed flexible probe during the insertion and compared it with the stiff probe during animal movement. Subsequently, we used this innovative technique to monitor the spinal glutamate signaling during myocardial ischemia and reperfusion that can cause fatal ventricular arrhythmias. We showed that glutamate concentration increases during the myocardial ischemia, persists during the reperfusion, and is associated with sympathoexcitation and increases in myocardial substrate excitability.
Keywords: arrhythmia; glutamate neurotransmitter; myocardial ischemia; real-time biosensing; spinal cord neural network.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


Update of
-
Real-time in vivo thoracic spinal glutamate sensing reveals spinal hyperactivity during myocardial ischemia.bioRxiv [Preprint]. 2023 Mar 24:2023.03.11.531911. doi: 10.1101/2023.03.11.531911. bioRxiv. 2023. Update in: Am J Physiol Heart Circ Physiol. 2023 Dec 1;325(6):H1304-H1317. doi: 10.1152/ajpheart.00299.2023 PMID: 36993301 Free PMC article. Updated. Preprint.
Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article.
-
"I've Spent My Whole Life Striving to Be Normal": Internalized Stigma and Perceived Impact of Diagnosis in Autistic Adults.Autism Adulthood. 2023 Dec 1;5(4):423-436. doi: 10.1089/aut.2022.0066. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116050 Free PMC article.
-
"It Is a Big Spider Web of Things": Sensory Experiences of Autistic Adults in Public Spaces.Autism Adulthood. 2023 Dec 1;5(4):411-422. doi: 10.1089/aut.2022.0024. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116051 Free PMC article.
-
Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
-
Improving Sensitivity and Longevity of In Vivo Glutamate Sensors with Electrodeposited NanoPt.ACS Appl Mater Interfaces. 2024 Aug 7;16(31):40570-40580. doi: 10.1021/acsami.4c06692. Epub 2024 Jul 30. ACS Appl Mater Interfaces. 2024. PMID: 39078097 Free PMC article.
-
Improving the Biocompatibility and Functionality of Neural Interface Devices with Silica Nanoparticles.Acc Chem Res. 2024 Jun 18;57(12):1684-1695. doi: 10.1021/acs.accounts.4c00160. Epub 2024 May 30. Acc Chem Res. 2024. PMID: 38814586 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

