Microglia in neurodegenerative diseases: mechanism and potential therapeutic targets
- PMID: 37735487
- PMCID: PMC10514343
- DOI: 10.1038/s41392-023-01588-0
Microglia in neurodegenerative diseases: mechanism and potential therapeutic targets
Abstract
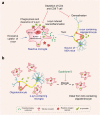
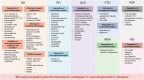
Microglia activation is observed in various neurodegenerative diseases. Recent advances in single-cell technologies have revealed that these reactive microglia were with high spatial and temporal heterogeneity. Some identified microglia in specific states correlate with pathological hallmarks and are associated with specific functions. Microglia both exert protective function by phagocytosing and clearing pathological protein aggregates and play detrimental roles due to excessive uptake of protein aggregates, which would lead to microglial phagocytic ability impairment, neuroinflammation, and eventually neurodegeneration. In addition, peripheral immune cells infiltration shapes microglia into a pro-inflammatory phenotype and accelerates disease progression. Microglia also act as a mobile vehicle to propagate protein aggregates. Extracellular vesicles released from microglia and autophagy impairment in microglia all contribute to pathological progression and neurodegeneration. Thus, enhancing microglial phagocytosis, reducing microglial-mediated neuroinflammation, inhibiting microglial exosome synthesis and secretion, and promoting microglial conversion into a protective phenotype are considered to be promising strategies for the therapy of neurodegenerative diseases. Here we comprehensively review the biology of microglia and the roles of microglia in neurodegenerative diseases, including Alzheimer's disease, Parkinson's disease, multiple system atrophy, amyotrophic lateral sclerosis, frontotemporal dementia, progressive supranuclear palsy, corticobasal degeneration, dementia with Lewy bodies and Huntington's disease. We also summarize the possible microglia-targeted interventions and treatments against neurodegenerative diseases with preclinical and clinical evidence in cell experiments, animal studies, and clinical trials.
© 2023. West China Hospital, Sichuan University.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
The Role of Microglia in Retinal Neurodegeneration: Alzheimer's Disease, Parkinson, and Glaucoma.Front Aging Neurosci. 2017 Jul 6;9:214. doi: 10.3389/fnagi.2017.00214. eCollection 2017. Front Aging Neurosci. 2017. PMID: 28729832 Free PMC article. Review.
-
Secondary Protein Aggregates in Neurodegenerative Diseases: Almost the Rule Rather than the Exception.Front Biosci (Landmark Ed). 2023 Oct 20;28(10):255. doi: 10.31083/j.fbl2810255. Front Biosci (Landmark Ed). 2023. PMID: 37919089 Review.
-
Targeting microglial autophagic degradation in NLRP3 inflammasome-mediated neurodegenerative diseases.Ageing Res Rev. 2021 Jan;65:101202. doi: 10.1016/j.arr.2020.101202. Epub 2020 Nov 5. Ageing Res Rev. 2021. PMID: 33161129 Review.
-
Motor neuron TDP-43 proteinopathy in progressive supranuclear palsy and corticobasal degeneration.Brain. 2022 Aug 27;145(8):2769-2784. doi: 10.1093/brain/awac091. Brain. 2022. PMID: 35274674
-
Role of neuroinflammation in neurodegeneration development.Signal Transduct Target Ther. 2023 Jul 12;8(1):267. doi: 10.1038/s41392-023-01486-5. Signal Transduct Target Ther. 2023. PMID: 37433768 Free PMC article. Review.
Cited by
-
Porphyromonas gingivalis triggers microglia activation and neurodegenerative processes through NOX4.Front Cell Infect Microbiol. 2024 Oct 14;14:1451683. doi: 10.3389/fcimb.2024.1451683. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39469453 Free PMC article.
-
Current Trends in Stroke Biomarkers: The Prognostic Role of S100 Calcium-Binding Protein B and Glial Fibrillary Acidic Protein.Life (Basel). 2024 Oct 1;14(10):1247. doi: 10.3390/life14101247. Life (Basel). 2024. PMID: 39459548 Free PMC article. Review.
-
A Human Brain-Chip for Modeling Brain Pathologies and Screening Blood-Brain Barrier Crossing Therapeutic Strategies.Pharmaceutics. 2024 Oct 10;16(10):1314. doi: 10.3390/pharmaceutics16101314. Pharmaceutics. 2024. PMID: 39458643 Free PMC article.
-
Clinical and Genetic Profiles of 5q- and Non-5q-Spinal Muscular Atrophy Diseases in Pediatric Patients.Genes (Basel). 2024 Sep 30;15(10):1294. doi: 10.3390/genes15101294. Genes (Basel). 2024. PMID: 39457418 Free PMC article. Review.
-
The Dual Role of Amyloid Beta-Peptide in Oxidative Stress and Inflammation: Unveiling Their Connections in Alzheimer's Disease Etiopathology.Antioxidants (Basel). 2024 Oct 8;13(10):1208. doi: 10.3390/antiox13101208. Antioxidants (Basel). 2024. PMID: 39456461 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

