SRC and TKS5 mediated podosome formation in fibroblasts promotes extracellular matrix invasion and pulmonary fibrosis
- PMID: 37735172
- PMCID: PMC10514346
- DOI: 10.1038/s41467-023-41614-x
SRC and TKS5 mediated podosome formation in fibroblasts promotes extracellular matrix invasion and pulmonary fibrosis
Abstract
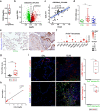
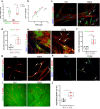
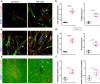
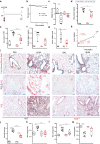
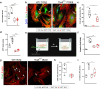
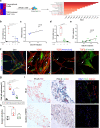
The activation and accumulation of lung fibroblasts resulting in aberrant deposition of extracellular matrix components, is a pathogenic hallmark of Idiopathic Pulmonary Fibrosis, a lethal and incurable disease. In this report, increased expression of TKS5, a scaffold protein essential for the formation of podosomes, was detected in the lung tissue of Idiopathic Pulmonary Fibrosis patients and bleomycin-treated mice. Τhe profibrotic milieu is found to induce TKS5 expression and the formation of prominent podosome rosettes in lung fibroblasts, that are retained ex vivo, culminating in increased extracellular matrix invasion. Tks5+/- mice are found resistant to bleomycin-induced pulmonary fibrosis, largely attributed to diminished podosome formation in fibroblasts and decreased extracellular matrix invasion. As computationally predicted, inhibition of src kinase is shown to potently attenuate podosome formation in lung fibroblasts and extracellular matrix invasion, and bleomycin-induced pulmonary fibrosis, suggesting pharmacological targeting of podosomes as a very promising therapeutic option in pulmonary fibrosis.
© 2023. Springer Nature Limited.
Conflict of interest statement
A.T. has received fees for speaking and/or organizing education from AstraZeneca, Menarini, Boehringer Ingelheim, Chiesi, Hoffmann-La Roche, Ltd., GlaxoSmithKline and Elpen, for consulting from Boehringer Ingelheim, Pfizer, Gilead, Hoffmann-La Roche, Ltd., GlaxoSmithKline, and has received research funding, including institutional funding, from Boehringer Ingelheim, Chiesi, Hoffmann-La Roche, Ltd., GlaxoSmithKline and Astra Zeneca, outside the submitted work. B.C. has received fees for speaking and/or organizing education from Apellis, Astra Zeneca, BMS, Boehringer Ingelheim, Novartis, Roche and Sanofi, for consulting fees from Apellis, BMS, Boehringer Ingelheim and Sanofi, and has received research funding from Boehringer Ingelheim, outside the submitted work. N.K. is a scientific founder at Thyron, served as a consultant to Biogen Idec, Boehringer Ingelheim, Third Rock, Pliant, Samumed, NuMedii, Theravance, LifeMax, Three Lake Partners, Optikira, Astra Zeneca, RohBar, Veracyte, Augmanity, CSL Behring, Galapagos and Thyron over the last 3 years, reports Equity in Pliant and Thyron, and grants from Veracyte, Boehringer Ingelheim, BMS and non-financial support from MiRagen and Astra Zeneca, outside the submitted work. Other authors declare that they have no conflict of interest.
Figures


Similar articles
-
A role for the podosome/invadopodia scaffold protein Tks5 in tumor growth in vivo.Eur J Cell Biol. 2008 Sep;87(8-9):555-67. doi: 10.1016/j.ejcb.2008.02.008. Epub 2008 Apr 15. Eur J Cell Biol. 2008. PMID: 18417249 Free PMC article.
-
The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells.Cancer Cell. 2005 Feb;7(2):155-65. doi: 10.1016/j.ccr.2005.01.006. Cancer Cell. 2005. PMID: 15710328
-
Invadosome Formation by Lung Fibroblasts in Idiopathic Pulmonary Fibrosis.Int J Mol Sci. 2022 Dec 28;24(1):499. doi: 10.3390/ijms24010499. Int J Mol Sci. 2022. PMID: 36613948 Free PMC article.
-
The SRC substrate Tks5, podosomes (invadopodia), and cancer cell invasion.Cold Spring Harb Symp Quant Biol. 2005;70:167-71. doi: 10.1101/sqb.2005.70.014. Cold Spring Harb Symp Quant Biol. 2005. PMID: 16869750
-
Advances in Understanding TKS4 and TKS5: Molecular Scaffolds Regulating Cellular Processes from Podosome and Invadopodium Formation to Differentiation and Tissue Homeostasis.Int J Mol Sci. 2020 Oct 30;21(21):8117. doi: 10.3390/ijms21218117. Int J Mol Sci. 2020. PMID: 33143131 Free PMC article. Review.
Cited by
-
Hypoxia Promotes Invadosome Formation by Lung Fibroblasts.Cells. 2024 Jul 6;13(13):1152. doi: 10.3390/cells13131152. Cells. 2024. PMID: 38995003 Free PMC article.
-
Microneedle delivery system with rapid dissolution and sustained release of bleomycin for the treatment of hemangiomas.J Nanobiotechnology. 2024 Jun 25;22(1):372. doi: 10.1186/s12951-024-02557-7. J Nanobiotechnology. 2024. PMID: 38918811 Free PMC article.
-
The axis of tumor-associated macrophages, extracellular matrix proteins, and cancer-associated fibroblasts in oncogenesis.Cancer Cell Int. 2024 Oct 7;24(1):335. doi: 10.1186/s12935-024-03518-8. Cancer Cell Int. 2024. PMID: 39375726 Free PMC article. Review.
-
Erythropoietin Reduces Inflammation, Oxidative Stress, and Apoptosis in a Rat Model of Bleomycin-Induced Idiopathic Pulmonary Fibrosis.J Pers Med. 2024 Sep 13;14(9):972. doi: 10.3390/jpm14090972. J Pers Med. 2024. PMID: 39338226 Free PMC article.
-
Fibroblast Activation Protein Alpha (FAPα) in Fibrosis: Beyond a Perspective Marker for Activated Stromal Cells?Biomolecules. 2023 Nov 29;13(12):1718. doi: 10.3390/biom13121718. Biomolecules. 2023. PMID: 38136590 Free PMC article. Review.
References
-
- Rockey DC, Bell PD, Hill JA. Fibrosis — a common pathway to organ injury and failure. N. Engl. J. Med. 2015;372:1138–1149. - PubMed
-
- Raghu G, et al. Diagnosis of idiopathic pulmonary fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018;198:e44–e68. - PubMed
-
- Martinez FJ, et al. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Prim. 2017;3:17074. - PubMed
-
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002;3:349–363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

