Light-inducible T cell engagers trigger, tune, and shape the activation of primary T cells
- PMID: 37722050
- PMCID: PMC10523538
- DOI: 10.1073/pnas.2302500120
Light-inducible T cell engagers trigger, tune, and shape the activation of primary T cells
Abstract
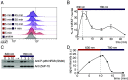
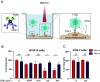
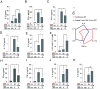
To mount appropriate responses, T cells integrate complex sequences of receptor stimuli perceived during transient interactions with antigen-presenting cells. Although it has been hypothesized that the dynamics of these interactions influence the outcome of T cell activation, methodological limitations have hindered its formal demonstration. Here, we have engineered the Light-inducible T cell engager (LiTE) system, a recombinant optogenetics-based molecular tool targeting the T cell receptor (TCR). The LiTE system constitutes a reversible molecular switch displaying exquisite reactivity. As proof of concept, we dissect how specific temporal patterns of TCR stimulation shape T cell activation. We established that CD4+ T cells respond to intermittent TCR stimulation more efficiently than their CD8+ T cells counterparts and provide evidence that distinct sequences of TCR stimulation encode different cytokine programs. Finally, we show that the LiTE system could be exploited to create light-activated bispecific T cell engagers and manipulate tumor cell killing. Overall, the LiTE system provides opportunities to understand how T cells integrate TCR stimulations and to trigger T cell cytotoxicity with high spatiotemporal control.
Keywords: T cell activation; TCR; bispecific T cell engagers; immunology; optogenetics.
Conflict of interest statement
Patent application PCT/EP2019/076914 and EP22305545.0 corresponding respectively to the LiTE and the LiTE-Me systems have been filed.
Figures



Similar articles
-
Programmable half-life and anti-tumour effects of bispecific T-cell engager-albumin fusions with tuned FcRn affinity.Commun Biol. 2021 Mar 8;4(1):310. doi: 10.1038/s42003-021-01790-2. Commun Biol. 2021. PMID: 33686177 Free PMC article.
-
Chronic TCR-MHC (self)-interactions limit the functional potential of TCR affinity-increased CD8 T lymphocytes.J Immunother Cancer. 2019 Nov 5;7(1):284. doi: 10.1186/s40425-019-0773-z. J Immunother Cancer. 2019. PMID: 31690351 Free PMC article.
-
CD4 and CD8 accessory molecules function through interactions with major histocompatibility complex molecules which are not directly associated with the T cell receptor-antigen complex.Eur J Immunol. 1991 Oct;21(10):2507-15. doi: 10.1002/eji.1830211030. Eur J Immunol. 1991. PMID: 1915555
-
Probing the activation requirements for naive CD8+ T cells with Drosophila cell transfectants as antigen presenting cells.Immunol Rev. 1998 Oct;165:249-65. doi: 10.1111/j.1600-065x.1998.tb01243.x. Immunol Rev. 1998. PMID: 9850865 Review.
-
Antigen-nonspecific activation of CD8+ T lymphocytes by cytokines: relevance to immunity, autoimmunity, and cancer.Arch Immunol Ther Exp (Warsz). 2008 Sep-Oct;56(5):311-23. doi: 10.1007/s00005-008-0033-2. Arch Immunol Ther Exp (Warsz). 2008. PMID: 18836862 Review.
References
-
- Hugues S., et al. , Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat. Immunol. 5, 1235–1242 (2004). - PubMed
-
- Mempel T. R., Henrickson S. E., Von Andrian U. H., T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 (2004). - PubMed
-
- Moreau H. D., Bousso P., Visualizing how T cells collect activation signals in vivo. Curr. Opin. Immunol. 26, 56–62 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

